glucose → glukoza
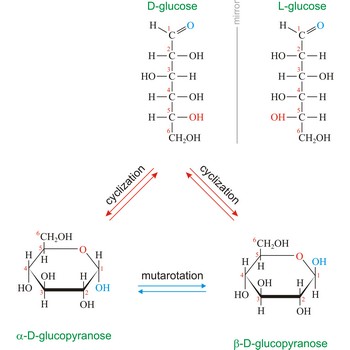
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
halogens → halogeni elementi
Halogens are the elements fluorine (F) chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metals, and make up part of the 17 group in the periodic table. Compounds of these elements are called halogenides or halides.
The halogens all have a strong unpleasant odour and will burn flesh. They do not dissolve well in water. The five elements are strongly electronegative. They are oxidising agents, with fluorine being the strongest and astatine being the weakest. They react with most metals and many non-metals.
Halogens form molecules which consist of atoms covalently bonded. With increasing atomic weight there is a gradation in physical properties. For example: Fluorine is a pale green gas of low density. Chlorine is a greenish-yellow gas 1.892 times as dense as fluorine. Bromine is a deep reddish-brown liquid which is three times as dense as water. Iodine is a grayish-black crystalline solid with a metallic appearance. And astatine is a solid with properties which indicate that it is somewhat metallic in character.
hemiacetal → poluacetal
Hemiacetals are organic compounds having the general formula R2C(OH)OR’ (R’ ≠ H), derived from aldehydes or ketones by formal addition of an alcohol to the carbonyl group. Hemiacetals are generally unstable compounds. In some cases however, stable cyclic hemiacetals can be readily formed, especially when 5- and 6-membered rings are possible. In this case an intramolecular OH group reacts with the carbonyl group. Glucose and many other aldoses exist as cyclic hemiacetals whereas fructose and similar ketoses exist as cyclic hemiketals. Originally, the term was confined to derivatives of aldehydes (one R = H), but it now applies equally to derivatives of ketones (neither R = H).
Hesse’s law → Hessov zakon
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
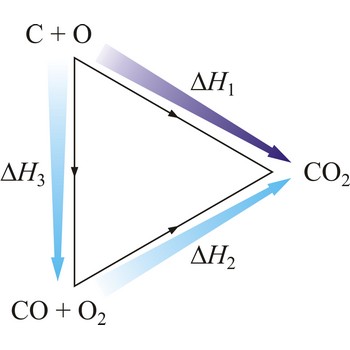
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
homologous series → homologni niz
Series of compounds which have a common general formula and in which each member differs from the next member by a constant unit, which is the methylene group (-CH2-) is called the homologous series. Members of a homologous series are called homolog.
An example of the homologous series with some of their homologs are given below. Straight chain alkanes having general formula CnH2n+2
| Structure | Name |
|---|---|
| CH4 | methane |
| CH3-CH3 | ethane |
| CH3-CH2-CH3 | propane |
| CH3-CH2CH2CH3 | butane |
| CH3-(CH2)3-CH3 | pentane |
| CH3-(CH2)4-CH3 | hexane |
| CH3-(CH2)5-CH3 | heptane |
| CH3-(CH2)6-CH3 | octane |
| CH3-(CH2)7-CH3 | nonane |
| CH3-(CH2)8-CH3 | decane |
hybrid orbital → hibridne orbitale
Hybrid orbital is an orbital created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals. For example, a common hybridization is sp3 where s orbital combine with a three p orbitals to form four new orbitals. After hybridization, all hybrid orbitals have the same energy, lower than p orbitals, but higher than s orbitals.
hydrosphere → hidrosfera
Hydrosphere (from the Greek for water sphere) is a discontinuous layer of water on, under, and over the Earth's surface. It includes all liquid and frozen surface waters, groundwater held in soil and rock, and atmospheric water vapour. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun.
| Reservoir | V / 106 km3 | w / % |
|---|---|---|
| oceans | 1 370.0 | 97.25 |
| ice caps and glaciers | 29.0 | 2.05 |
| groundwater | 9.5 | 0.68 |
| lakes, rivers | 0.127 | 0.01 |
| soil moisture | 0.065 | 0.005 |
| atmosphere (as liquid equivalent of water vapour) | 0.013 | 0.001 |
| biosphere | 0.0006 | 0.00004 |
| TOTAL | 1 408.7 | 100 |
lanthanides → lantanoidi
Lanthanides (lanthanons, lanthanoids or rare-earth elements) are a series of fourteen elements in the periodic table, generally considered to range in proton number from cerium to lutetium inclusive. It was convenient to divide these elements into the cerium group or light earth: cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu); and the yttrium group or heavy earths: gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) i lutetium (Lu). The position of lanthanum is somewhat equivocal and, although not itself a lanthanide, it is often included with them for comparative purpose. The lanthanides are sometimes simply called the rare earths. Apart from unstable Pm, the lanthanides are actually not rare. Cerium is the 26. most abundant of all elements, 5 times as abundant as Pb. All are silvery very reactive metals.
lime → živo vapno
Lime (or quicklime) is the common name for calcium oxide (CaO). It is manufactured from limestone, CaCO3, by heating it to a high temperature (about 1 000 °C). At this temperature carbon dioxide, CO2, is released from the limestone creating calcium oxide, CaO.
A further process involves adding water in a process known as hydrating, which produces hydrated, or slaked lime [Ca(OH)2].
Citing this page:
Generalic, Eni. "Create table if not exists postgresql." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table