tantalum → tantal
Tantalum was discovered by Anders Ekeberg (Sweden) in 1802. The origin of the name comes from the Greek word Tantalos meaning father of Niobe in Greek mythology, (tantalum is closely related to niobium in the periodic table). It is rare, grey, heavy, hard but ductile, metal with a high melting point. Exposed surfaces form corrosion resistant oxide film. Attacked by HF and fused alkalis. Metal ignites in air. Tantalum always found with niobium. Chiefly occurs in the mineral tantalite. Often used as an economical substitute for platinum. Tantalum pentoxide is used in capacitors and in camera lenses to increase refracting power. It and its alloys are corrosion and wear resistant so it is used to make surgical and dental tools.
titar → titar
Titar (T) is a mass of titrated matter which is equivalent to 1 cm3 of solution. It is shown as T = 2.356 mg HCl / 1.0 cm3 NaOH, 0.1000 moldm-3, and it is usually shown in a table form. If the concentration of used standard solution (c) differs from one outlined in the table data (c0), the factor of correction (f) is induced
Titar is usually used in industrial operational laboratories where from titar tables mass or percentage of the ingredient in question is directly read.
Torricelli, Evangelista → Torricelli, Evangelista
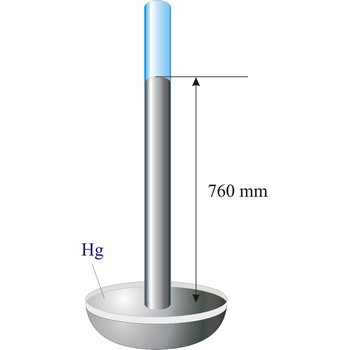
Evangelista Torricelli (1852-1908) is Italian physicist and mathematician. He became the first scientist to create a sustained vacuum and to discover the principle of a barometer. He filled a tube three feet long, and hermetically closed at one end, with mercury and set it vertically with the open end in a basin of mercury, taking care that no air-bubbles should get into the tube. The column of mercury invariably fell to about twenty-eight inches, leaving an empty space above its level. He discovered that the variation of the height of the mercury from day to day was caused by changes in the atmospheric pressure. He also constructed a number of large objectives and small, short focus, simple microscopes.
unsaturated fatty acid → nezasićena masna kiselina
Unsaturated fatty acid is a fatty acid whose carbon chain can absorb additional hydrogen atoms. Their carbon chain has one or more double or triple valence bond per molecule. The most important of these are:
| Oleic (9-octadecenoic acid) | CH3(CH2)7CH=CH(CH2)7COOH |
| Linoleic (9,12-octadecadienoic acid) | CH3(CHCH2)3(CH2CH=CH)2(CHCH2)7COOH |
| Linolenic (9,12,15-octadecatrienoic acid) | CH3(CH2CH=CH)3(CHCH2)7COOH |
visible radiation → vidljivo zračenje
Human eye can only see electromagnetic radiation of wavelengths form 400 nm to 760 nm. This narrow part of electromagnetic spectrum is called visible radiation. Visible (white) light is a mixture of light of all kind of colours, it can be separated, with the help of a glass prism, into its component colours - visible light spectrum, and each colour corresponds to a certain area of wavelengths:
| Colour | Wavelength / nm |
|---|---|
| purple | 400 - 450 |
| blue | 450 - 500 |
| green | 500 - 570 |
| yellow | 570 - 590 |
| orange | 590 - 620 |
| red | 620 - 760 |
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
zwitterion → dipolarni ion
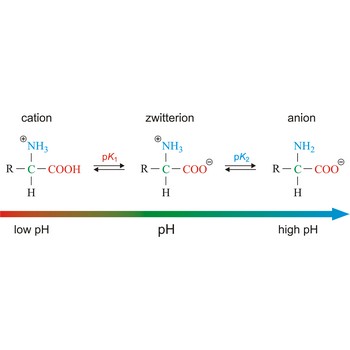
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
water jet vacuum pump → vodena sisaljka
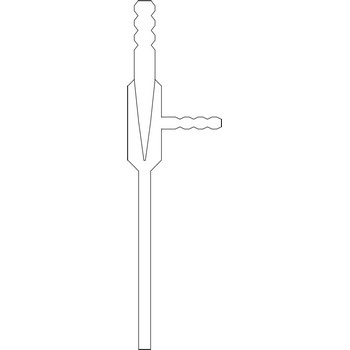
The water jet vacuum pump or vacuum aspirator is one of the most popular devices that produces vacuum in laboratories. The rapid flow of water through the device creates a vacuum in a side-arm that is connected to a vacuum application such a Buchner flask. The water jet vacuum pump creates a vacuum by means of Venturi effect named after the Italian physicist Giovanni Battista Venturi (1746–1822). The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. Water jet pumps are manufactured of glass, plastic or metal, depending on the conditions in which they are used.
Citing this page:
Generalic, Eni. "Create table if not exists postgresql." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table