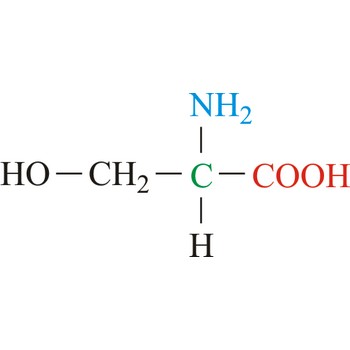
isoleucine → izoleucin
Isoleucine is hydrophobic amino acids with aliphatic side chain. It is one of the three amino acids having branched hydrocarbon side chains. The side chains of these amino acids are not reactive but, these residues are critically important for ligand binding to proteins, and play central roles in protein stability. Isoleucine is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Ile, I
- IUPAC name: 2-amino-3-methylpentanoic acid
- Molecular formula: C6H13NO2
- Molecular weight: 131.12 g/mol
lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
lysine → lizin
Lysine is an electrically charged amino acids with basic side chains. Lysine is a base, as are arginine and histidine. The amino group is highly reactive and often participates in reactions at the active centers of enzymes. Lysine plays an important role in coordinating negatively charged ligands. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Lys, K
- IUPAC name: 2,6-diaminohexanoic acid
- Molecular formula: C6H14N2O2
- Molecular weight: 146.19 g/mol
nerve poison → živčani bojni otrov
Nerve poison (nerve gas, agents) have had an entirely dominant role since the Second World War. Nerve poisons acquired their name because they affect the transmission of nerve impulses in the nervous system. All nerve poisons belong chemically to the group of organo-phosphorus compounds. They are stable and easily dispersed, highly toxic and have rapid effects both when absorbed through the skin and via respiration. Nerve poisons can be manufactured by means of fairly simple chemical techniques. The raw materials are inexpensive and generally readily available.
The most important nerve agents included in modern chemical weapons arsenals are:
| Tabun | (o-ethyl dimethylamidophosphorylcyanide) |
| Sarin | (isopropyl methylphosphonofluoridate) |
| Soman | (pinacolyl methylphosphonofluoridate) |
| GF | (cyclohexyl methylphosphonofluoridate) |
| VX | (o-ethyl S-diisopropylaminomethyl methylphosphonothiolate) |
Nerve poisons are colorless, odorless, tasteless liquids of low volatility. Antidotes are atropine sulfate and pralidoxime iodide.
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
ribonucleic acid → ribonukleinska kiselina
Ribonucleic acid is a complex organic compound in living cells that is concerned with protein synthesis. Plays an intermediary role in converting the information contained in DNA into proteins. RNA carries the genetic information from DNA to those parts of the cell where proteins are made. Some viruses store their genetic information as RNA not as DNA.
Ribonucleic acid is a similar molecule to DNA but with a slightly different structure.
The structural difference with DNA is that RNA contains a -OH group both at the 2' and 3' position of the ribose ring, whereas DNA (which stands, in fact, for deoxy-RNA) lacks such a hydroxy group at the 2' position of the ribose. The same bases can be attached to the ribose group in RNA as occur in DNA, with the exception that in RNA thymine does not occur, and is replaced by uracil, which has an H-group instead of a methyl group at the C-5 position of the pyrimidine. Unlike the double-stranded DNA molecule, RNA is a single-stranded molecule.
The three main functionally distinct varieties of RNA molecules are: (1) messenger RNA (mRNA) which is involved in the transmission of DNA information, (2) ribosomal RNa (rRNA) which makes up the physical machinery of the synthetic process, and (3) transfer RNA (tRNA) which also constitutes another functional part of the machinery of protein synthesis.
serine → serin
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
supercritical fluid extraction → superkritična fluidna ekstrakcija
Supercritical fluid extractions (SFE) have solvating powers similar to liquid organic solvents, but with higher diffusivities, lower viscosity, and lower surface tension. The main advantages of using supercritical fluids for extractions is that they are inexpensive, contaminant free, and less costly to dispose safely than organic solvents. For non-destructive isolation choose SFE, which is simply the best technology for sensitive raw materials. For these reasons supercritical carbon dioxide (scCO2) is the reagent used to extract caffeine from coffee and tea. Its gaslike behavior allows it to penetrate deep into the green coffee beans, and it dissolves from 97 % to 99 % of the caffeine present.
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
Citing this page:
Generalic, Eni. "Cost per roll." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table