Acheson process → Achesonov proces
Acheson process is an industrial process to synthesize graphite and silicon carbide (carborundum), named after its inventor the American chemist Edward Goodrich Acheson (1856-1931). In this process, a solid-state reaction between pure silica sand (SiO2) and petroleum coke (C) at very high temperature (more than 2500 °C) leads to the formation of silicon carbide under the general reaction:
While studying the effects of high temperature on carborundum, Acheson had found that silicon vaporizes at about 4150 °C, leaving behind graphitic carbon.
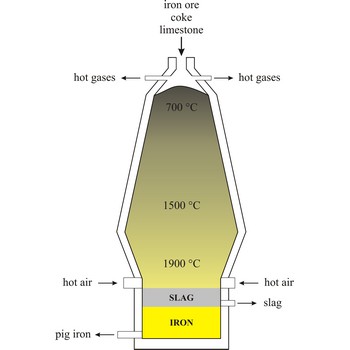
blast furnace → visoka peć
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
coal gas → ugljeni plin
Coal gas is a gas produced by the destructive distillation of coal, and contains approximately 50 % hydrogen, 35 % methane and 8 % carbon monoxide. The by-products of the production of coal gas are coal tar and coke.
dioxin → dioksin
Dioxin is a general term that describes a group of hundreds of chemicals that are highly persistent in the environment. The most toxic compound is 2,3,7,8-tetrachlorodibenzo-p-dioxin or TCDD. The toxicity of other dioxins and chemicals like PCBs that act like dioxin are measured in relation to TCDD. Dioxin is formed as an unintentional by-product of many industrial processes involving chlorine such as waste incineration, chemical and pesticide manufacturing and pulp and paper bleaching. Dioxin was the primary toxic component of Agent Orange, found at Love Canal in Niagara Falls, NY and was the basis for evacuations at Times Beach, MO and Seveso, Italy.
Dioxin is formed by burning chlorine-based chemical compounds with hydrocarbons. The major source of dioxin in the environment comes from waste-burning incinerators of various sorts and also from backyard burn-barrels. Dioxin pollution is also affiliated with paper mills which use chlorine bleaching in their process, with the production of Polyvinyl Chloride (PVC) plastics, and with the production of certain chlorinated chemicals (like many pesticides).
graphite → grafit
Graphite is an allotrope of carbon. The atoms are arranged in layers as a series of flat, hexagonal rings. Graphite is a good conductor of heat and electricity. The layers cleave easily, making graphite useful as a solid lubricant. A process to make pure synthetic graphite was invented by the American chemist Edward Goodrich Acheson (1856–1931). The process consists of heating a mixture of clay (aluminum silicate) and powdered coke (carbon) in an iron bowl. The reaction involves the production of silicon carbide, which loses silicon at 4150 °C to leave graphite.
iron → željezo
Iron has been known since ancient times. The origin of the name comes from the Latin word ferrum meaning iron. It is malleable, ductile, silvery-white metal. Exposed surfaces form red-brown oxides. Forms very strong alloys (steel). Ferromagnetic. Metal dust flammable. Fourth most abundant element in the earth’s crust. Iron is obtained from iron ores. Pure metal produced in blast furnaces by layering limestone, coke and iron ore and forcing hot gasses into the bottom. This heats the coke red hot and the iron is reduced from its oxides and liquefied where it flows to the bottom. Iron is the most common metal in human society. More than 90 % of all metal refined in the world is iron. Used in steel and other alloys. It is the chief constituent of hemoglobin which carries oxygen in blood vessels. Its oxides are used in magnetic tapes and disks.
phosphorus → fosfor
Phosphorus was discovered by Hennig Brandt (Germany) in 1669. The origin of the name comes from the Greek word phosphoros meaning bringer of light. White phosphorus is white to yellow soft, waxy phosphorescent solid with acrid fumes. Toxic by inhalation, ingestion, or skin contact. Red phosphorus is powdery, non-flammable and non-toxic. Phosphorus is found most often in phosphate rock. Pure form is obtained by heating a mixture of phosphate rock, coke and silica to about 1450 °C. Used in the production of fertilizers and detergents. Some is used in fireworks, safety matches and incendiary weapons. Phosphorus is also important in the production of steels, phosphor bronze and many other products.
tellurium → telurij
Tellurium was discovered by Franz Joseph Muller von Reichstein (Romania) in 1782. The origin of the name comes from the Latin word tellus meaning earth. It is silvery-white, brittle semi-metal. Unreactive with water or HCl; dissolves in HNO3; burns in air or oxygen. Tellurium is obtained as a by-product of copper and lead refining. Used to improve the machining quality of copper and stainless steel products and to colour glass and ceramics. Also in thermoelectric devices. Some is used in the rubber industry and it is a basic ingredient in manufacturing blasting caps.
Citing this page:
Generalic, Eni. "Coke manufacturing." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table