amount concentration → količinska koncentracija
Amount concentration (also called molar concentration and in older literature molarity) is the amount of a given substance in a stated unit of a mixture, solution, or ore. The common unit is mole per cubic decimetre (moldm−3) or mole per litre (molL-1) sometimes denoted by M.
The concentration of an atom, ion, or molecule in a solution may be symbolised by the use of square brackets, as [Ca2+].
angular velocity → kutna brzina
A point-like object that undergoes circular motion changes its angular position from initial Θi to final Θf, relative to a fixed axis, specified in a coordinate system with an origin that coincides the centre of the circular path of object. The change in its angular position is called angular displacement ΔΘ = Θf - Θi. Also, a rigid body that rotates about a specified rotation axis, changing its angular position from initial Θi to final Θf, undergoes an angular displacement ΔΘ.
The average angular velocity, ωav, is the ratio of the angular displacement and the time interval Δt=tf-ti, in which that displacement occurs.
Θf and Θi are the initial and final angular position, respectively.
The instantaneous angular velocity ω is the limit of the average angular velocity, as Δt is made to approach zero.
ωav and ω are positive for the counterclockwise rotation (in direction of increasing Θ) and negative for the clockwise rotation (in direction of decreasing Θ).
SI unit for angular velocity is s-1.The measure for the angle Θ is radian. The relationship between radians and degrees is:
For example, the angular velocity of the minute hand of a clock is:
average rate of reaction → prosječna brzina reakcije
Average rate of reaction is calculated in a way that a total change of reactants and products concentration is divided with time which is needed for reaction to end.
Boltzmann equation → Boltzmannova jednadžba
Boltzmann equation is a statistical definition of entropy, given by
where S and k are the entropy and Boltzmann’s constant, respectively, and W is the probability of finding the system in a particular state.
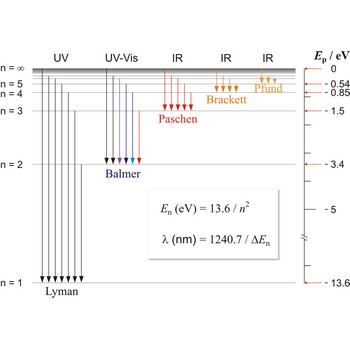
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
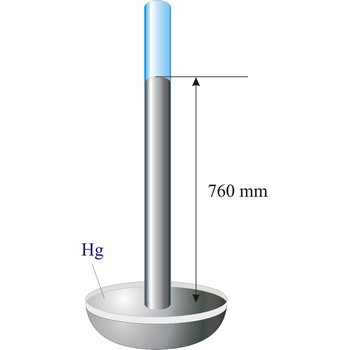
barometer → barometar
Barometer is an instrument that measures atmospheric pressure. A mercury barometer is a closed tube filled with mercury inverted in a mercury reservoir. The height of the mercury column indicates atmospheric pressure (with 1 atm = 760 mm of mercury). An aneroid barometer consists of an evacuated container with a flexible wall. When atmospheric pressure changes, the wall flexes and moves a pointer which indicates the changing pressure on a scale.
biocapacity → biokapacitet
Biocapacity (or biological capacity) is the capacity of ecosystems to produce useful biological materials and to absorb carbon dioxide generated by humans, using current management schemes and extraction technologies. Useful biological materials are defined as those used by the human economy, hence what is considered useful can change from year to year. The biocapacity of an area is calculated by multiplying the actual physical area by the yield factor and the appropriate equivalence factor.
Yield factor is a factor that accounts for differences between countries in productivity of a given land type. Each country and each year has yield factors for cropland, grazing land, forest, and fisheries.
Equivalence factor is a productivity based scaling factor that converts a specific land type into a universal unit of biologically productive area, a global hectare (gha).
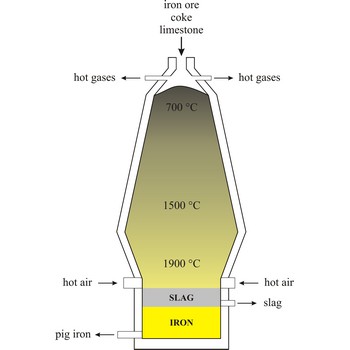
blast furnace → visoka peć
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
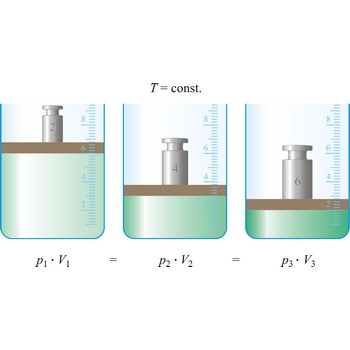
Boyle’s law → Boyleov zakon
Boyle’s law (sometimes referred to as the Boyle-Mariott’s law) is the empirical law, exact only for an ideal gas, which states that the volume of a gas is inversely proportional to its pressure at constant temperature.
Citing this page:
Generalic, Eni. "Change of state." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table