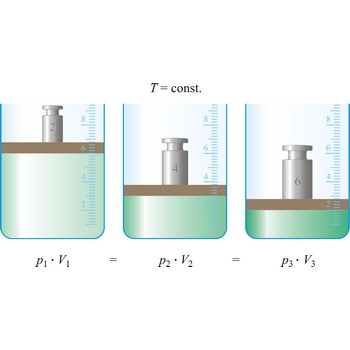
Boyle’s law (sometimes referred to as the Boyle-Mariott’s law) is the empirical law, exact only for an ideal gas, which states that the volume of a gas is inversely proportional to its pressure at constant temperature.
Citing this page:
Generalic, Eni. "Boyle’s law." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table
Find in dictionary:
Copyright © 2004-2023 by Eni Generalic. All rights reserved. | Disclaimer