cryogenic fractionation → kriogena frakcinacija
Cryogenic fractionation is a process of separation of gases by cooling them until they enter their liquid state. Large scale gas production companies use this method to produce liquid oxygen, liquid nitrogen etc. Gases have different boiling points (the temperature at which they change from liquid to gas). Oxygen has a boiling point of -183 °C, and nitrogen a boiling point of -195.8 °C. Therefore by cooling the gas mixture to -183 °C, the oxygen can be collected as liquid and the nitrogen remains its gaseous form.
freezing → smrzavanje
Freezing is the change of a liquid into a solid state as the temperature decreases. For water, the freezing point is 0 °C (or 273.16 K).
heat of fusion → toplina taljenja
Heat of fusion or enthalpy of fusion is the heat required to convert a substance from the solid to the liquid state with no temperature change (also called latent heat of fusion or melting).
heat of vaporisation → toplina isparavanja
Heat of vaporisation or enthalpy of vaporisation is the heat required to convert a substance from the liquid to the gaseous state with no temperature change (also called latent heat of vaporization).
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
Gibbs phase rule → Gibbsov zakon faza
Gibbs phase rule is the relationship used to determine the number of state variables, usually chosen from among temperature, pressure, and species composition in each phase, which must be specified to fix the thermodynamic state of a system in equilibrium:
where C is the number of components in a mixture, P is the number of phases, and F is the degrees of freedom, i.e., the number of intensive variables that can be changed independently without affecting the number of phases.
global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
Hesse’s law → Hessov zakon
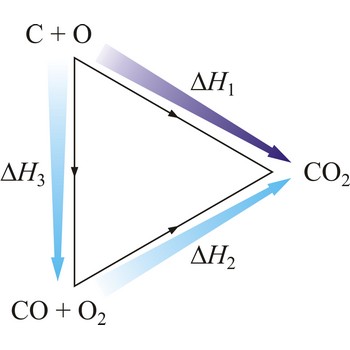
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
law of conservation of mass → zakon o očuvanju mase
Law of conservation of mass states that no detectable gain or loss in mass occurs in chemical reactions. The state of a substance may change in a chemical reaction, for example, from a solid to a gas, but its total mass will not change. Note that the energy released (exothermic) or adsorbed (endothermic) in a chemical reaction is a result of energy transfer between atoms and their environment.
valence electron → valentni elektron
Valence electrons are electrons that can be actively involved in a chemical change, usually electrons in the outermost (valent) shell. For example, sodium’s ground state electron configuration is 1s2 2s2 2p6 3s1; the 3s electron is the only valence electron in the atom. Germanium (Ge) has the ground state electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2; the 4s and 4p electrons are the valence electrons.
Citing this page:
Generalic, Eni. "Change of state." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table