blackbody → crno tijelo
In radiation physics, an ideal blackbody is a theoretical object that absorbs all the radiant energy falling upon it and emits it in the form of thermal radiation. Planck’s radiation law gives the power radiated by a unit area of blackbody, and the Stefan-Boltzman law expresses the total power radiated.
blackbody radiation → zračenje crnog tijela
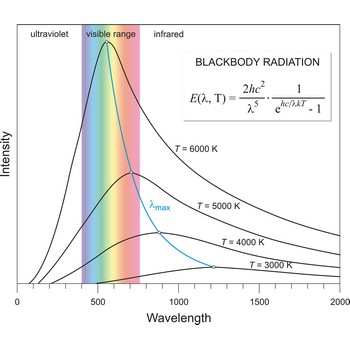
Blackbody radiation is the radiation emitted by a perfect blackbody, i.e., a body which absorbs all radiation incident on it and reflects none. The primary law governing blackbody radiation is the Planck Radiation Law, which governs the intensity of radiation emitted by unit surface area into a fixed direction (solid angle) from the blackbody as a function of wavelength for a fixed temperature. The Planck Law can be expressed through the following equation
where λ is the wavelength, h is Planck’s constant, c is the speed of light, k is the Boltzmann constant, and T is the temperature.
allotrope → alotrop
Allotropes are the elements which exist in two or more different forms in the same physical state. Allotropes generally differ in physical properties and may also differ in chemical activity.
Diamond, graphite and fullerenes are three allotropes of the element carbon. Graphite is a soft, black, slippery substance; by contrast, diamond is one of the hardest substances known. The different properties of the allotropes arise from their chemical structures. Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Graphite crystallizes in the hexagonal system. In the fullerenes, the carbon atoms taking the form of a hollow sphere, ellipsoid, or tube.
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C.
The term allotropes may also be used to refer to the molecular forms of an element. Ozone is a chemically active triatomic allotrope of the element oxygen.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
blanching → blanširanje
1. Blanching is a heat treatment of foodstuffs to partially or completely inactivate the naturally occurring enzymes prior to freezing.
2. Blanching is a washing process for coins cleaning. The black surface layer of cupric oxide is removing by dipping the coins in hot dilute sulphuric acid (w(H2SO4) = 10 %).
analytical balance → analitička vaga
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
Citing this page:
Generalic, Eni. "Black comedians in corpus christi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table