thallium → talij
Thallium was discovered by Sir William Crookes (England) in 1861. The origin of the name comes from the Greek word thallos meaning green twig or green shoot. It is soft grey metal that looks like lead. Tarnishes in moist air. Reacts in heated moist air and in acids. Compounds highly toxic by inhalation or ingestion. Cumulative effects. Thallium is found in iron pyrites. Also in crookesite, hutchinsonite and lorandite. Most is recovered from the by-products of lead and zinc refining. Its compounds are used in rat and ant poisons. Also for detecting infrared radiation.
thin layer chromatography → tankoslojna kromatografija
Thin layer chromatography. (TLC) is a technique for separating components in a mixture on the basis of their differing polarities. A spot of sample is placed on a flat sheet coated with silica and then carried along by a solvent that soaks the sheet. Different components will move different distances over the surface. TLC is a useful screening technique in clinical chemistry; for example, it can be used to detect the presence of drugs in urine.
tin → kositar
Tin has been known since ancient times. The origin of the name comes from the Latin word stannum meaning tin. It is silvery-white, soft, malleable and ductile metal. Exposed surfaces form oxide film. Resists oxygen and water. Dissolves in acids and bases. Organic tin compounds may be highly toxic. Tin is principally found in the ore cassiterite (SnO2) and stannine (Cu2FeSnS4). Used as a coating for steel cans since it is non-toxic and non-corrosive. Also in solder (33 %Sn:67 %Pb), bronze (20 %Sn:80 %Cu) and pewter. Stannous fluoride (SnF2), a compound of tin and fluorine is used in some toothpaste.
triols → trioli
Trihydric alcohols (i.e. Triols) are organic compounds containing three hydroxyl groups. The simplest trihydric alcohol is 1,2,3-propane-triol, CH2(OH)CH(OH)CH2(OH), which is also known as glycerol (from the Greek glykys meaning sweet) or glycerin. Glycerol is commercially produced by the hydrolysis of fats.
Glycerol is a by-product in the soap industry and is recovered by suitable means.
xenon → ksenon
Xenon was discovered by Sir William Ramsay, Morris W. Travers (England) in 1898. The origin of the name comes from the Greek word xenos meaning stranger. It is heavy, colourless, odourless, noble gas. Reacts only with fluorine. Xenon is obtain from the small quantities in liquid air. Used for filling flash lamps and other powerful lamps. Electrical excitation of xenon produces a burst of brilliant white light. Also used in bubble chambers and modern nuclear power reactors.
zirconium → cirkonij
Zirconium was discovered by Martin Heinrich Klaproth (Germany) in 1789. The origin of the name comes from the Arabic word zargun meaning gold colour. It is grey-white, lustrous, corrosion-resistant metal. Exposed surfaces form oxide protective film. Zirconium is found in many minerals such as zircon and baddeleyite. Used in alloys such as zircaloy this is used in nuclear applications since it does not readily absorb neutrons. Also baddeleyite is used in lab crucibles. Used in high-performance pumps and valves. Clear zircon (ZrSiO4) is a popular gemstone.
zwitterion → dipolarni ion
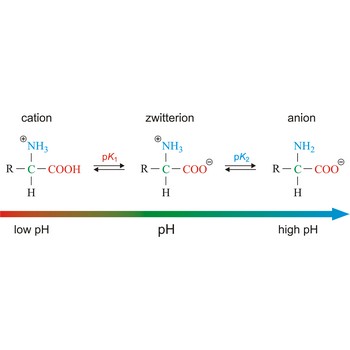
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Citing this page:
Generalic, Eni. "Balance carried forward meaning." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table