absolute error → apsolutna pogreška
Absolute error is a difference between the obtained value (O) and the real value (μ). It is shown in employed for measuring (g, cm3, ...). For example, if three replicate weights for an object are 1.00 g, 1.05 g, and 0.95 g, the absolute error can be expressed as ±0.05 g. Absolute error is also used to express inaccuracies; for example, if the "true value" is 1.11 g and the measured value is 1.00 g, the absolute error could be written as 1.00 g - 1.11 g = -0.11 g. Note that when absolute errors are associated with indeterminate errors, they are preceded by "±"; when they are associated with determinate errors, they are preceded by their sign.
absolute temperature → apsolutna temperatura
Absolute temperature denoting a temperature measured on the absolute scale, a scale of temperature based on absolute zero as the lowest temperature.
absolute volume → apsolutni volumen
Absolute volume is the total volume of the particles in a granular material, including both permeable and impermeable voids but excluding spaces between particles.
absolute zero → apsolutna nula temperature
Absolute zero is theoretically, the lowest attainable temperature. It is the energy at which the kinetic energy of atom and molecules is minimal and is equivalent to -273.15 °C.
barometer → barometar
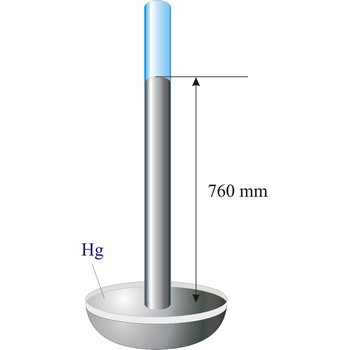
Barometer is an instrument that measures atmospheric pressure. A mercury barometer is a closed tube filled with mercury inverted in a mercury reservoir. The height of the mercury column indicates atmospheric pressure (with 1 atm = 760 mm of mercury). An aneroid barometer consists of an evacuated container with a flexible wall. When atmospheric pressure changes, the wall flexes and moves a pointer which indicates the changing pressure on a scale.
Charles’ law → Charlesov zakon
The volume of a fixed mass of gas at a constant pressure expand by the constant fraction of its volume at 0 °C. For each Celsius or kelvin degree its temperature is raised. For any ideal gas fraction it is approximately 1/273. This can be expressed by the equation
were V° is the volume at 0°C and V is its volume at t°C.
This is equivalent to the statement that the volume of a fixed mass of gas at a constant pressure is proportional to its thermodynamic temperature
This law also know as Gay-Lussac’s law.
An equation similar to the one given above applies to pressures for ideal gases:
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
entropy → entropija
Entropy (S) is a measure of the unavailability of a system’s energy to do work; in a closed system, an increase in entropy is accompanied by a decrease in energy availability. When a system undergoes a reversible change the entropy (S) changes by an amount equal to the energy (Q) transferred to the system by heat divided by the thermodynamic temperature (T) at which this occurs.
All real processes are to a certain extent irreversible changes and in any closed system an irreversible change is always accompanied by an increase in entropy.
lattice energy → energija kristalne rešetke
Lattice energy is the energy per ion pair required to separate completely the ions in a crystal lattice at a temperature of absolute zero.
Citing this page:
Generalic, Eni. "Apsolutno." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table