accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
angular momentum → moment količine gibanja
Angular momentum is a physical quantity defined for rotating motion (in analogy to momentum that is defined for linear motion). If a body rotates around a specified axis, its angular momentum equals
Where I is the rotational inertia concerning that axis and ω is the angular velocity of the body.
Angular momentum can also be defined for a point-like body concerning a specified origin (in that case, it is not necessary that the point-like body undergoes circular motion). Rotational inertia of the point-like body, concerning that origin equals:
Where m is the mass of the body and r is its distance from the origin.
antimony → antimon
Antimony has been known since ancient times. The origin of the name comes from the Latin word stibium meaning mineral stibnite. It is hard, brittle, silvery-white semimetal. Stable in dry air. Toxic by ingestion or inhalation. Antimony is found in stibnite (Sb2S3) and in valentinite (Sb2O3). It is alloyed with other metals to increase their hardness. Also in the manufacture of a few special types of semiconductor devices. Also in plastics and chemicals. A few kinds of over-the-counter cold and flu remedies use antimony compounds.
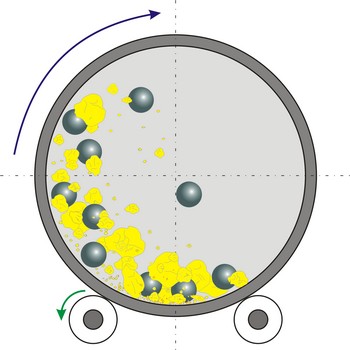
ball mill → kuglični mlin
Ball mill is a grinder for reducing hard materials to powder. The grinding is carried out by the pounding and rolling of a charge of steel or ceramic balls carried within the cylinder. The cylinder rotates at a relatively slow speed, allowing the balls to cascade through the mill base, thus grinding or dispersing the materials.
Type of ball mills, centrifugal and planetary mills, are devices used to rapidly grind materials to colloidal fineness (approximately 1 μm and below) by developing high grinding energy via centrifugal and/or planetary action.
chemical technology → kemijska tehnologija
Chemical technology is a branch of applied chemistry that concerns technical methods and devices in order to manufacture a chemical product.
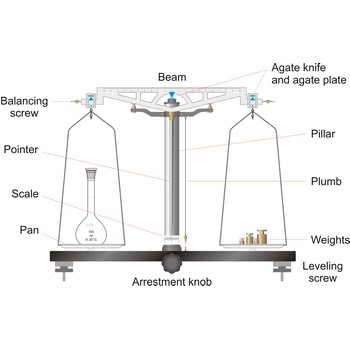
balance → vaga
Balance is an instrument to measure the mass (or weight) of a body. Balance beam type scales are the oldest type and measure weight using a fulcrum or pivot and a lever with the unknown weight placed on one end of the lever, and a counterweight applied to the other end. When the lever is balanced, the unknown weight and the counterweight are equal. The equal-arm balance consists of two identical pans hung from either end of a centrally suspended beam. The unequal-arm balance is made with one arm of the balance much longer than the other.
More modern substitution balances use the substitution principle. In this calibrated weights are removed from the single lever arm to bring the single pan suspended from it into equilibrium with a fixed counter weight. The substitution balance is more accurate than the two-pan device and enables weighing to be carried out more rapidly.
Electromagnetic force restoration balances also use a lever system but a magnetic field is used to generate the force on the opposite end of the lever and balance out the unknown mass. The current used to drive the magnetic coil is proportional to the mass of the object placed on the platform.
battery → baterija
Battery a device that converts chemical energy to electrical energy. The process underlying the operation of a battery involves a chemical reaction in which electrons are transferred from one chemical species to another. This process is carried out in two half-reactions, one that involves the loss of electrons and one that involves their gain. The battery is an electrochemical cell divided in two half-cells, and reaction proceeds when these are connected together by an electrically conducting pathway. The passage of electrons from one half-cell to the other corresponds to an electric current. Each half-cell contains an electrode in contact with the reacting species. The electrode which passes electrons into the circuit when battery discharges is called anode and is negative terminal. The electrode which receives electrons is called cathode, and is the battery’s positive terminal. The electrical circuit is completed by an electrolyte, an electrically conducting substance placed between the two electrodes which carriers a flow of charge between them. In wet cells, the electrolyte is a liquid containing dissolved ions, whose motion generates an electrical current; in dry cells the electrolyte is basely solid, for example, a solid with mobile ions or porous solid saturated with an ionic solution.
gas thermometer → plinski termometar
Gas thermometer is a device for measuring temperature in which the working fluid is a gas.
Citing this page:
Generalic, Eni. "Analog devices LT7176." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table