haematite → hematit
Haematite is a mineral of iron(III) oxide Fe2O3. It is the most important ore of iron and usually occurs in two main forms: as a massive red kidney-shaped ore and as grey to black metallic crystals known as specular iron ore. Haematite is the major red colouring agent in rocks; the largest deposits are of sedimentary origin. In industry haematite is also used as a polishing agent (jeweller’s rouge) and in paints.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
hafnium → hafnij
Hafnium was discovered by Dirk Coster (Denmark) and Georg Karl von Hevesy (Hungary) in 1923. The origin of the name comes from the Latin name Hafnia meaning Copenhagen. It is silvery, ductile metal. Exposed surfaces form oxide film. Resists alkalis and acids (except HF). Toxic. Metal ignites and burns readily. Hafnium is obtained from mineral zircon or baddeleyite. Used in reactor control rods because of its ability to absorb neutrons.
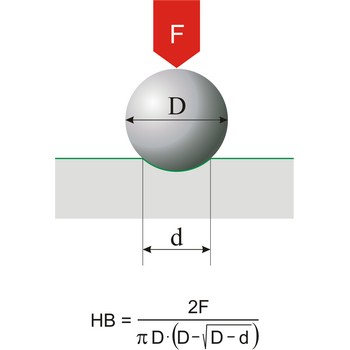
hardness → tvrdoća
Hardness is the resistance of a material to deformation of an indenter of specific size and shape under a known load. This definition applies to all types of hardness scales except Mohs scale, which is a based on the concept of scratch hardness and is used chiefly for minerals. The most generally used hardness scales are Brinell (for cast iron), Rockwell (for sheet metal and heat-treated steel), Knoop (for metals).
helium → helij
Helium was discovered by Pierre Jules César Janssen (France) and Sir William Ramsay (Scotland) in 1868. The origin of the name comes from the Greek word helios meaning sun. It is light, odourless, colourless inert gas. Second most abundant element in the universe. Helium is found in natural gas deposits from wells in Texas, Oklahoma and Kansas. Used in balloons, deep sea diving and welding. Also used in very low temperature research.
hemiacetal → poluacetal
Hemiacetals are organic compounds having the general formula R2C(OH)OR’ (R’ ≠ H), derived from aldehydes or ketones by formal addition of an alcohol to the carbonyl group. Hemiacetals are generally unstable compounds. In some cases however, stable cyclic hemiacetals can be readily formed, especially when 5- and 6-membered rings are possible. In this case an intramolecular OH group reacts with the carbonyl group. Glucose and many other aldoses exist as cyclic hemiacetals whereas fructose and similar ketoses exist as cyclic hemiketals. Originally, the term was confined to derivatives of aldehydes (one R = H), but it now applies equally to derivatives of ketones (neither R = H).
international system of units → međunarodni sustav jedinica
International System of Units (SI) is the unit system adopted by the General Conference on Weights and Measures in 1960 and recommended for use in all scientific and technical fields. It consists of seven base units (meter, kilogram, second, ampere, kelvin, mole, candela), plus derived units and prefixes.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
mass → masa
Mass (m) is the quantity of matter contained in a particle or body regardless of its location in the universe. Mass is constant, whereas weight is affected by the distance of a body from the centre of the Earth (or of other planet). The SI unit is kilogram.
According to the Einstein equation
all forms of energy possess a mass equivalent.
Citing this page:
Generalic, Eni. "Alicikli�ki spojevi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table