activated complex → aktivirani kompleks
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
complexometry → kompleksometrija
Complexometry is a volumetric analytic method which is based on titration of metal ion solutions with a substance that, combined with metal ions yields complex compounds, e.g. EDTA. The stoichiometric ratio of EDTA-metal in complexometric analyses is always 1:1, whatever the valency of the metal
activated charcoal → aktivni ugljen
Activated charcoal or activated carbon is charcoal that has been activated for adsorption by steaming or by heating in a vacuum. Charcoal is obtained by burning wood, nutshells, coconut husks or other materials. Charcoal becomes activated by heating it with steam to approximately 1000 °C in the absence of oxygen.
The chemical nature of amorphous carbon, combined with a high surface area makes it an ideal medium for the adsorption of organic chemicals. A single gram of such material can have 400 m2 to 1 200 m2 square meters of surface area. Activated charcoal is widely used to decolorize liquids, recover solvents, and remove toxins from water and air.
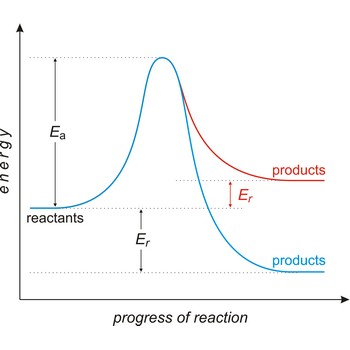
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
aluminate → aluminat
Aluminate is a salt formed when aluminium hydroxide or y-alumina is dissolved in solutions of strong bases, such as sodium hydroxide. Aluminates exist in solutions containing the aluminate ion, commonly written [Al(OH)4]-. In fact the ion probably is a complex hydrated ion and can be regarded as formed from a hydrated Al3+ ion by removal of four hydrogen ions:
Other aluminates and polyaluminates, such as [Al(OH)6]3- and [(HO)3AlOAl(OH)3]2-, are also present.
chelating agent → kelatni agens
Chelating agent is ligand that binds to a metal using more than one atom; a polydentate ligand.
cyanide process → cijanidni postupak
Cyanide process is a method for separating a metal from an ore. Crushed ore is treated with cyanide ion to produce a soluble metal cyanide complex. The complex is washed out of the ore and reduced to metallic form using an active metal (usually zinc).
ionophore → ionofore
Ionophore is a relatively small hydrophobic molecule that facilitates the transport of ions across lipid membranes. Most ionophores are produced by microorganisms. There are two types of ionophores: channel formers, which combine to form a channel in the membrane through which ions can flow; and mobile ion carriers, which transport ions across a membrane by forming a complex with the ion.
ligand field theory → teorija ligandnog polja
Ligand field theory is a description of the structure of crystals containing a transition metal ion surrounded by nonmetallic ions (ligands). It is based on the construction of molecular orbitals involving the d-orbitals of the central metal ion and combinations of atomic orbitals of the ligands.
Citing this page:
Generalic, Eni. "Aktivirani kompleks." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table