phosphorus → fosfor
Phosphorus was discovered by Hennig Brandt (Germany) in 1669. The origin of the name comes from the Greek word phosphoros meaning bringer of light. White phosphorus is white to yellow soft, waxy phosphorescent solid with acrid fumes. Toxic by inhalation, ingestion, or skin contact. Red phosphorus is powdery, non-flammable and non-toxic. Phosphorus is found most often in phosphate rock. Pure form is obtained by heating a mixture of phosphate rock, coke and silica to about 1450 °C. Used in the production of fertilizers and detergents. Some is used in fireworks, safety matches and incendiary weapons. Phosphorus is also important in the production of steels, phosphor bronze and many other products.
platinum → platina
Platinum was discovered by Antonio de Ulloa (South America) in 1735. The origin of the name comes from the Spanish word platina meaning silver. It is rare, very heavy, soft, silvery-white metal. Resists oxygen and water. Platinum is produced from deposits of native, or elemental. Used in jewellery, to make crucible and special containers and as a catalyst. Used with cobalt to produce very strong magnets. Also to make standard weights and measures. Resists corrosion and acid attacks except aqua regia.
plutonium → plutonij
Plutonium was discovered by Glenn T. Seaborg, Edwin M. McMillan, J. W. Kennedy and A. C. Wahl (USA) in 1940. Named after the planet Pluto. It is silvery-white, extremely radioactive artificially produced metal. Reacts with oxygen and acids; resists alkalis. Attacked by steam. Highly toxic. Plutonium is found rarely in some uranium ores. Made by bombarding uranium with neutrons. Used in bombs and reactors. Small quantities are used in thermo-electric generators.
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
potassium → kalij
Potassium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Arabic word qali meaning alkali (the origin of the symbol K comes from the Latin word kalium). It is soft, waxy, silver-white metal. Fresh surface has silvery sheen. Quickly forms dull oxide coating on exposure to air. Reacts strongly with water. Reacts with water to give off flammable gas. Reacts violently with oxidants. Occurs only in compounds. Potassium is found in minerals like carnallite [(KMgCl3)·6H2O] and sylvite (KCL). Pure metal is produced by the reaction of hot potassium chloride and sodium vapours in a special retort. Used as potash in making glass and soap. Also as saltpetre, potassium nitrate (KNO3) to make explosives and to colour fireworks in mauve. Vital to function of nerve and muscle tissues.
praseodymium → praseodimij
Praseodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words prasios didymos meaning green twin. It is silvery white, moderately soft, malleable, ductile metal. Reacts slowly with oxygen. Reacts rapidly with water. Metal ignites and burns readily. Praseodymium is obtained from same salts as neodymium. Used with neodymium to make lenses for glass maker’s goggles since it filters out the yellow light present in glass blowing. Alloyed with magnesium creates a high-strength metal used in aircraft engines. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 5 % praseodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4).
proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
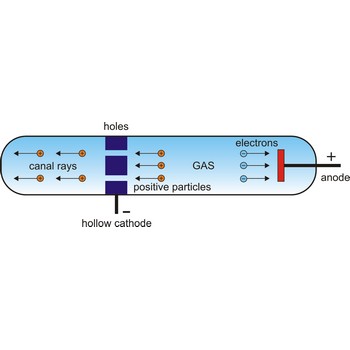
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
radium → radij
Radium was discovered by Marie and Pierre Curie (France) in 1898. The origin of the name comes from the Latin word radius meaning ray. It is silvery-white radioactive metal. Reacts with oxygen and water. Highly radiotoxic. Carcinogen by inhalation, ingestion, or exposure. Radium is found in uranium ores at 1 part per 3 million parts uranium. Used in treating cancer because of the gamma rays it gives off.
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
rhodium → rodij
Rhodium was discovered by William Hyde Wollaston (England) in 1804. The origin of the name comes from the Greek word rhodon meaning rose. It is hard, silvery-white metal. Inert in air and acids. Reacts with fused alkalis. Rhodium is obtained as a by-product of nickel production. Used as a coating to prevent wear on high quality science equipment and with platinum to make thermocouples.
Citing this page:
Generalic, Eni. "X-zrake." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table