practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
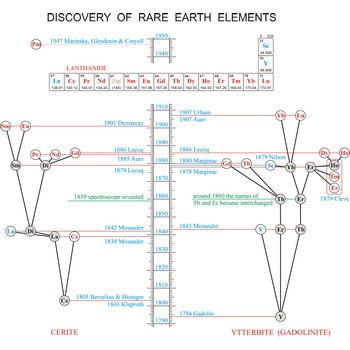
rare earth elements → elementi rijetkih zemalja
Rare earth elements (metals) are the elements scandium (Sc), yttrium (Y), and the lanthanides (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). These elements got their name from the fact that chemists first isolated them in their oxide forms. These oxides somewhat resemble calcium, magnesium and aluminium oxides, sometimes called common earths. Do you want to know more?
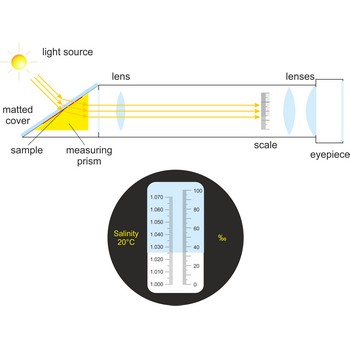
refractometer → refraktometar
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
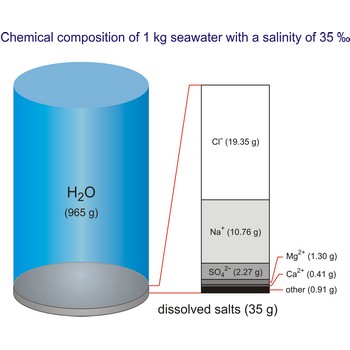
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
Schellbach’s burette → Schellbachova bireta
When colourless liquids are used, parallax mistake is avoided by use of Schellbach’s burette. On the inside wall opposite to graduation scale it has a melted in ribbon from milky glass in the middle of which a blue line is found. The level of liquid is now spotted very easily because of light breaking in the meniscus blue line now looks like a double spike.
seawater → more
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
thorium → torij
Thorium was discovered by Jöns Jakob Berzelius (Sweden) in 1828. Named after Thor, the mythological Scandinavian god of war. It is heavy, grey, soft, malleable, ductile, radioactive metal. Tarnishes in air; reacts with water. Reacts violently with oxidants. Thorium is found in various minerals like monazite and thorite. Used in making strong alloys. Also in ultraviolet photoelectric cells. It is a common ingredient in high-quality lenses. Bombarded with neutrons make uranium-233, a nuclear fuel.
thulium → tulij
Thulium was discovered by Per Theodore Cleve (Sweden) in 1879. Named after Thule, an ancient name for Scandinavia. It is soft, malleable, ductile, silvery metal. Tarnishes in air. Reacts with water. Flammable dust. Thulium is found with other rare earths in the minerals gadolinite, euxenite, xenotime and monazite. Radioactive thulium is used to power portable X-ray machines, eliminating the need for electrical equipment.
transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
unequal-arm balance → vaga s različitim krakovima
The lever principle on which these scales are constructed is based on the law of physics that at equilibrium the force applied at one end of the lever multiplied by the length of the arm (distance from the fulcrum to the point where the force is applied) must be equal to the product of the force acting at the opposite end of the lever and the length of the other arm.
The unequal-arm balance is preferred for work when large amounts are to be weighed.
Citing this page:
Generalic, Eni. "Kill to Survive scan vf." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table