Gauss’ law for electrostatics → Gaussov zakon za elektrostatiku
Gauss’ law describes the relation between charge and electric field in static situations, so it is equivalent to Coulomb’s law, which can be derived from Gauss’ law. Gauss’ law states that the net flux of electric field, Φ, through an imaginary closed surface, S, - a Gaussian surface - is equal to the net charge, q, inside that closed surface:
where electric flux Φ through Gaussian surface is given by:
ε0 is the permittivity constant and dS is a surface element.
Avogadro’s law → Avogadrov zakon
Avogadro’s law: Equal volumes of all gases contain equal numbers of molecules at the same pressure and temperature. The law, often called Avogadro’s hypothesis, is true only for ideal gases. It was proposed in 1811 by Italian chemist Amadeo Avogadro (1776-1856).
Beer’s law → Beerov zakon
Beer’s law (or Beer-Lambert law) is the functional relationship between the quantity measured in an absorption method (A) and the quantity sought, the analyte concentration (c). As a consequence of interactions between the photons and absorbing particles, the power of the beam is attenuated from Po to P. Beer’s law can be written
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Biot-Savart law → Biot-Savartov zakon
The magnetic field B due to a current-carrying conductor can be determined by Biot-Savart law. The contribution to magnetic field set up at distance r by the current element IdL is given by expression:
where μ0 is permeability constant. It plays a role in magnetic problems equivalent to the role of permittivity constant μ0 in electrostatics problems. In order to obtain B, contributions of all current elements have to be integrated. In case of a long straight conductor, carrying current I, Biot-Savart law gives:
SI unit for magnetic field B is tesla (T).
Permaeability constant μ0 has value 4π×10-7 T m A-1.
Fick’s law → Fickov zakon
Fick’s law is the statement that the flux J of a diffusing substance is proportional to the concentration gradient, i.e.,
where D is called the diffusion coefficient.
first law of thermo-dynamics → prvi zakon termodinamike
First law of thermo-dynamics is: Energy can be neither created nor destroyed, but can cross from one shape to another.
Gaussian system of units → Gaussov sustav jedinica
Gaussian system of units is a hybrid system used in electromagnetic theory, which combines features of both the electrostatic cgs subsystem (esu) and electromagnetic cgs subsystem (emu). With three base units, it uses em units in magnetism and es units in electrostatics. This involves using the constant c (the velocity of light in vacuum) to interrelate these sets of units.
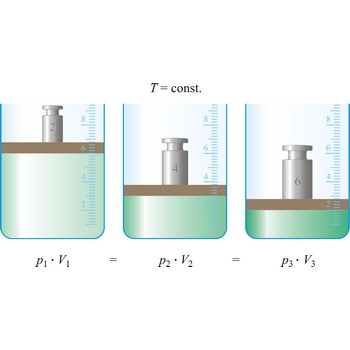
Boyle’s law → Boyleov zakon
Boyle’s law (sometimes referred to as the Boyle-Mariott’s law) is the empirical law, exact only for an ideal gas, which states that the volume of a gas is inversely proportional to its pressure at constant temperature.
Charles’ law → Charlesov zakon
The volume of a fixed mass of gas at a constant pressure expand by the constant fraction of its volume at 0 °C. For each Celsius or kelvin degree its temperature is raised. For any ideal gas fraction it is approximately 1/273. This can be expressed by the equation
were V° is the volume at 0°C and V is its volume at t°C.
This is equivalent to the statement that the volume of a fixed mass of gas at a constant pressure is proportional to its thermodynamic temperature
This law also know as Gay-Lussac’s law.
An equation similar to the one given above applies to pressures for ideal gases:
Citing this page:
Generalic, Eni. "Gaussov zakon za elektrostatiku." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table