samarium → samarij
Samarium was discovered by Paul Emile Lecoq de Boisbaudran (France) in 1879. Named after the mineral samarskite. It is silvery rare earth metal. Stable in dry air. Oxide coating forms on surfaces exposed to moist air. Metal ignites and burns readily. Reacts with water. Samarium is found with other rare earths in monazite sand. It is used in the electronics and ceramics industries. It is easily magnetized and very difficult to demagnetise. This suggests important future applications in solid-state and superconductor technologies.
sediment → sediment
1. Sediment is a fragmental material that originates from weathering of rocks and is transported by, suspended in, or deposited by water or air or is accumulated in beds by other natural agencies. When solidified, sediments form sedimentary rocks.
2. Strictly, sediment is a solid material that has settled down from a state of suspension in a liquid.
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
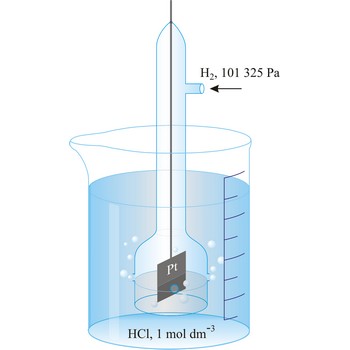
standard hydrogen electrode → standardna vodikova elektroda
Standard hydrogen electrode is a system in which hydrogen ion and gaseous hydrogen are present in their standard states. The convention is to designate the cell so that the standard hydrogen electrode is written first.
The electrode is used as a reference (of zero) for the values of other standard electrode potentials.
supercritical carbon dioxide → superkritični ugljikov dioksid
Supercritical carbon dioxide (scCO2) is a powerful, cheap, non-toxic and environmental friendly solvent. When used at a supercritical state (over 74 bar and 31 °C), it achieves similar solvating power as its organic competitors, such as hydrocarbons and chlorinated solvents. Supercritical carbon dioxide is one of few solvents that can be unrestrictedly used for food processing.
superfluid helium → superfluidni helij
Superfluidity in helium-4 was discovered in 1938 by the Soviet physicist Pyotr Leonidovich Kapitsa. Helium-4 exhibits superfluidity when it is cooled below 2.18 K (-270.97 C), which is called the lambda (λ) point. At these temperatures, helium-4 exhibits the characteristics of two distinct fluids, one of which appears to flow without friction. An extensive series of experiments showed that in this state of helium, called helium II (He II), there is an apparent enormous rise in heat conductivity, at an increase rate of about three million. Another unusual property of He II is its mobile, rapid flow through capillaries or over the rim of its containment vessel as a thin film that exhibits no measurable viscosity and appears unaffected by the forces of gravity or evaporation and condensation.
superoxide → superoksid
Superoxides are binary compounds containing oxygen in the -½ oxidation state. Sodium superoxide (NaO2) can be prepared with high oxygen pressures, whereas the superoxides of rubidium, potassium, and cesium can be prepared directly by combustion in air. These compounds are yellow to orange paramagnetic solids. Superoxide ion, O2-, has an unpaired electron, is not particularly stable, and spontaneously decomposes into peroxide over time.
They are strong oxidising agents that vigorously hydrolyze (react with water) to produce superoxide and oxygen gas.
terbium → terbij
Terbium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is soft, ductile, silvery-grey, rare earth metal. Oxidizes slowly in air. Reacts with cold water. Terbium is found with other rare earths in monazite sand. Other sources are xenotime and euxenite, both of which are oxide mixtures that can contain up to 1 % terbium. It is used in modest amounts in special lasers and solid-state devices.
transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
Citing this page:
Generalic, Eni. "50 states program guide." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table