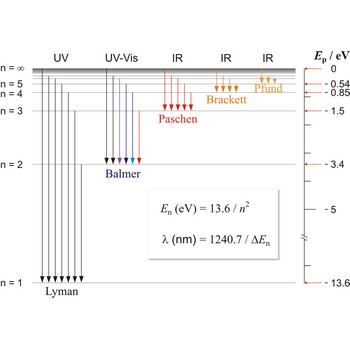
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
period → perioda
Periods are horizontal rows in the periodic table, each period begin with an alkali metal (one electron in the outermost principal quantum level) and ending with a noble gas (each having eight electrons in the outermost principal quantum level, except for helium, which is limited to two).
pH → pH
pH is a convenient measure of the acid-base character of a solution, usually defined by
where c(H+) is the concentration of hydrogen ions in moles per litre. The more precise definition is in terms of activity rather than concentration.
A solution of pH 0 to 7 is acid, pH of 7 is neutral, pH over 7 to 14 is alkaline.
phase diagram → fazni dijagram
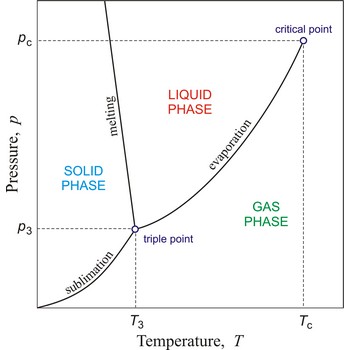
Phase diagram is a graphic representation of the equilibrium relationships between phases (such as vapour-liquid, liquid-solid) of a chemical compound, mixture of compounds, or solution.
The figure shows a typical phase diagram of an element or a simple compound. The stability of solid, liquid and gas phases depends on the temperature and the pressure. The three phases are in equilibrium at the triple point. The gas and liquid phases are separated by a phase transition only below the temperature of the critical point.
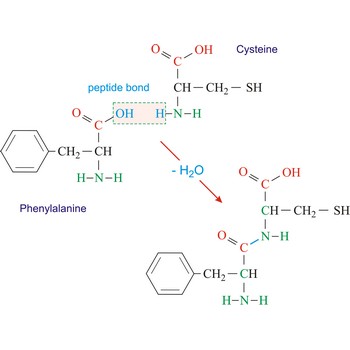
phenylalanine → fenilalanin
Phenylalanine is hydrophobic amino acids with aromatic side chain. It is quite hydrophobic and even the free amino acid is not very soluble in water. Phenylalanine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Phe, F
- IUPAC name: 2-amino-3-phenylpropanoic acid
- Molecular formula: C9H11NO2
- Molecular weight: 165.19 g/mol
phosphorus → fosfor
Phosphorus was discovered by Hennig Brandt (Germany) in 1669. The origin of the name comes from the Greek word phosphoros meaning bringer of light. White phosphorus is white to yellow soft, waxy phosphorescent solid with acrid fumes. Toxic by inhalation, ingestion, or skin contact. Red phosphorus is powdery, non-flammable and non-toxic. Phosphorus is found most often in phosphate rock. Pure form is obtained by heating a mixture of phosphate rock, coke and silica to about 1450 °C. Used in the production of fertilizers and detergents. Some is used in fireworks, safety matches and incendiary weapons. Phosphorus is also important in the production of steels, phosphor bronze and many other products.
plutonium → plutonij
Plutonium was discovered by Glenn T. Seaborg, Edwin M. McMillan, J. W. Kennedy and A. C. Wahl (USA) in 1940. Named after the planet Pluto. It is silvery-white, extremely radioactive artificially produced metal. Reacts with oxygen and acids; resists alkalis. Attacked by steam. Highly toxic. Plutonium is found rarely in some uranium ores. Made by bombarding uranium with neutrons. Used in bombs and reactors. Small quantities are used in thermo-electric generators.
pipette → pipeta
Pipettes are glass tubes which are tapers towards at both ends into narrow opened tubes. According to their design two types of pipettes can be distinguished:
Volumetric pipettes
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette
Graduated pipettes
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. They are filled in the same way as volumetric ones and liquid can be gradually released.
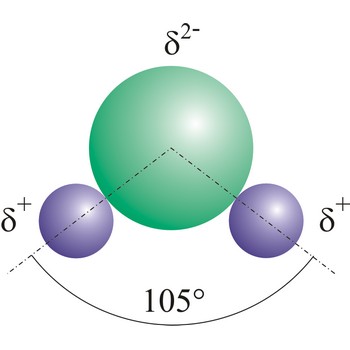
polar molecule → polarna molekula
Polar molecules are molecules at which centres of gravity of positive and negative charge are not in the same point.
Citing this page:
Generalic, Eni. "Voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table