triple point → trojna točka
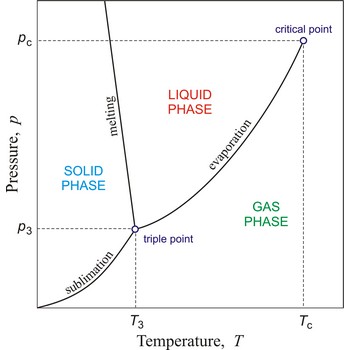
Triple point is the point in p,T space where the solid, liquid, and gas phases of a substance are in thermodynamic equilibrium.
critical point → kritična točka
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
equivalence point → točka ekvivalencije
Eequivalence point is the point in a titration when enough titrant has been added to react completely with the analyte.
isoelectric point → izoelektrična točka
Isoelectric point (pI or IEP) is the pH of a solution or dispersion at which the net charge on the molecules or colloidal particles is zero. In electrophoresis there is no motion of the particles in an electric field at the isoelectric point. The net charge (the algebraic sum of all the charged groups present) of any amino acid, peptide or protein, will depend upon the pH of the surrounding aqueous environment. For example, alanine can have a charge of +1, 0, or -1, depending on the pH of the solution in which it is dissolved.
point-like object → materijalna točka
Point-like object is an expression, usual in kinematics: a point-like object (or a particle) is an object with dimensions, which can be neglected while considering its motion.
kelvin → kelvin
Kelvin (K) is the SI base unit of thermodynamic temperature.
The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. The unit was named after the British scientist Sir. W. Thompson, Lord Kelvin (1824-1907).phase diagram → fazni dijagram
Phase diagram is a graphic representation of the equilibrium relationships between phases (such as vapour-liquid, liquid-solid) of a chemical compound, mixture of compounds, or solution.
The figure shows a typical phase diagram of an element or a simple compound. The stability of solid, liquid and gas phases depends on the temperature and the pressure. The three phases are in equilibrium at the triple point. The gas and liquid phases are separated by a phase transition only below the temperature of the critical point.
acceleration → akceleracija
If a point-like object undergoes a change in velocity Δv=vf-vi in time Δt=tf-ti (indexes i and f stand for initial and final instant as well as for initial and final velocity) its average acceleration, a is defined as
The instantaneous acceleration, a, is obtained from the average acceleration by shrinking the time interval Δt towards zero. The average acceleration approaches a limiting value, which is the acceleration of a given instant:
Acceleration is a vector quantity. SI unit for acceleration is m s-2.
acid-base titration → kiselo-bazna titracija
Acid-base titration is an analytical technique in volumetric analysis, where an acid of known concentration is used to neutralise a known volume of a base, and the observed volume of the acid required is used to determine the unknown concentration of the base. An acid-base indicator is used to determine the end-point of the titration.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
Citing this page:
Generalic, Eni. "Trojna točka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table