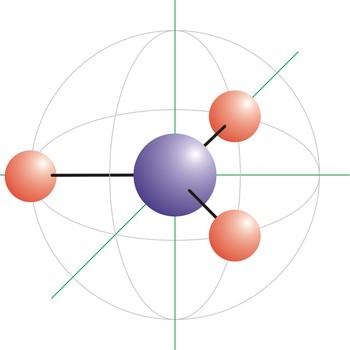
Trigonal planar is a molecular shape that results when there are three bonds and no lone pairs around the central atom in the molecule. The pairs are arranged along the central atom’s equator, with 120° angles between them. Molecules with an trigonal planar electron pair geometries have sp2d hybridization at the central atom. The carbonate ion (CO32-) has a trigonal planar geometry.
Citing this page:
Generalic, Eni. "Trigonal planar molecular geometry." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table
Find in dictionary:
Copyright © 2004-2023 by Eni Generalic. All rights reserved. | Disclaimer