plastic → plastika
Plastic is a material that can be shaped by the application of heat or pressure. Most are based on synthetic polymers although some are the product of natural substances (such as cellulose derivatives, but excluding the rubbers.). They are usually light and permanent solids, being also heat and electric isolators. If the materials soften again when reheated, they are said to be thermoplastic. If, after fashioning, they resist further applications of heat, they are said to be thermoset.
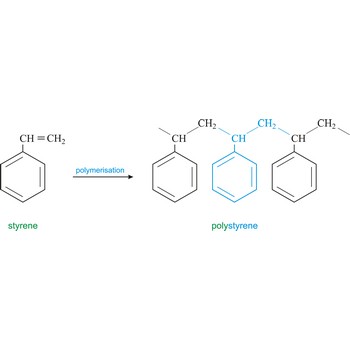
polymer → polimer
Polymer is a substance composed of molecules of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass (monomers). In most cases the number of monomers is quite large and is often not precisely known. A single molecule of a polymer is called a macromolecule. Polystyrene is light solid material obtained by polymerisation of styrene (vinyl benzene).
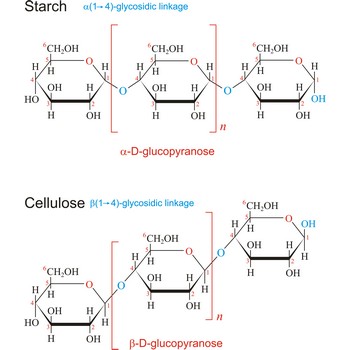
polysaccharide → polisaharid
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
potential energy → potencijalna energija
Potential energy (Ep) is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy). Gravitational potential energy is the energy associated with the state of separation between bodies that attracts each other via gravitational force. Elastic potential energy is the energy associated with the state of compression or extension of an elastic object. Thermal energy is associated with the random motions of atoms and molecules in a body.
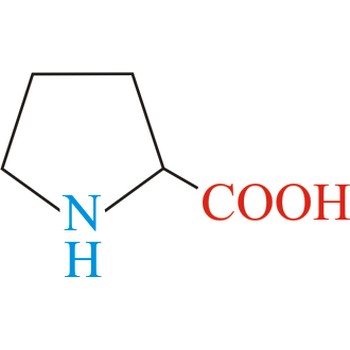
proline → prolin
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
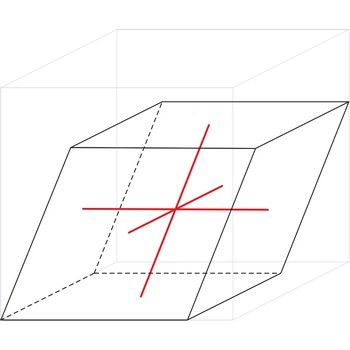
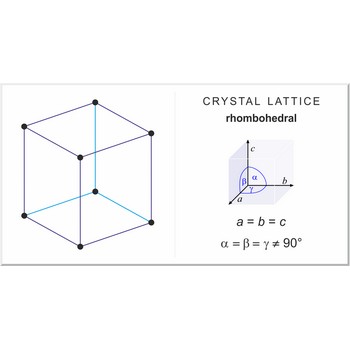
rhombohedral crystal system → romboedarski kristalni sustav
Rhombohedral crystal system is also known as the trigonal system. The crystallographic axes used in this system are of equal length. None of the axes are perpendicular to any other axis.
a = b = c
α= β = γ ≠ 90°
rhombohedral lattice → romboedarska rešetka
Rhombohedral (or trigonal) lattice has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b=c and interaxial angles α=β=γ≠90°.
serine → serin
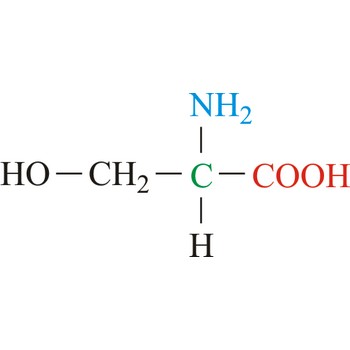
Serine is neutral amino acids with polar side chains. It is one of two hydroxyl amino acids. Both are commonly considered to by hydrophilic due to the hydrogen bonding capacity of the hydroxyl group. Serine often serves as a nucleophile in many enzyme active sites, and is best known for its role in the serine proteases. Serine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine.
- Abbreviations: Ser, S
- IUPAC name: 2-amino-3-hydroxypropanoic acid
- Molecular formula: C3H7NO3
- Molecular weight: 105.09 g/mol
solid state → čvrsto agregatno stanje
Solid state is characterised by a constant shape and volume. Particles are placed very close to one another and have efect one on another with great attraction forces. Solid bodies do not assume the shape of the container in which they are put.
standard → standard
Standards are materials containing a known concentration of an analyte. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
The accuracy of an analytical measurement is how close a result comes to the true value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. This is often done with standards of several concentrations to make a calibration or working curve.
A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has a high molecular weight.
A secondary standard is a standard that is prepared in the laboratory for a specific analysis. It is usually standardised against a primary standard.
Citing this page:
Generalic, Eni. "Trigonal bipyramidal molecular shape." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table