haematite → hematit
Haematite is a mineral of iron(III) oxide Fe2O3. It is the most important ore of iron and usually occurs in two main forms: as a massive red kidney-shaped ore and as grey to black metallic crystals known as specular iron ore. Haematite is the major red colouring agent in rocks; the largest deposits are of sedimentary origin. In industry haematite is also used as a polishing agent (jeweller’s rouge) and in paints.
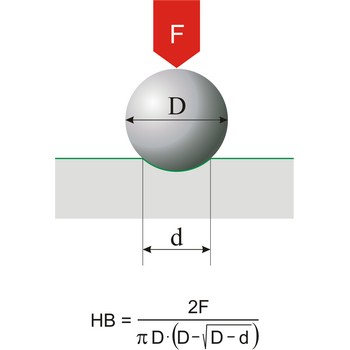
hardness → tvrdoća
Hardness is the resistance of a material to deformation of an indenter of specific size and shape under a known load. This definition applies to all types of hardness scales except Mohs scale, which is a based on the concept of scratch hardness and is used chiefly for minerals. The most generally used hardness scales are Brinell (for cast iron), Rockwell (for sheet metal and heat-treated steel), Knoop (for metals).
histidine → histidin
Histidine is an electrically charged amino acids with basic side chains. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Histidine is perhaps the most common and versatile catalytic residue in proteins. The imidazole sidechain of histidine has a pKa of approximately 6.0. This means that, at physiologically relevant pH values, relatively small shifts in pH will change its average charge. The unprotonated imidazole is nucleophilic and can serve as a general base, while the protonated form can serve as a general acid. In addition, it is often a ligand for transition metal ions such as iron and zinc.
- Abbreviations: His, H
- IUPAC name: 2-amino-3-(1H-imidazol-5-yl)propanoic acid
- Molecular formula: C6H9N3O2
- Molecular weight: 155.15 g/mol
percentage composition of atom in the molecule → postotni sastav atoma u molekuli
Percentage composition of atom in the molecule is a structure of compound presented in the shape of a percentage of its mass, which comes from every element.
plastic sulphur → plastični sumpor
Plastic sulphur is the shape of sulphur which emerges when hot, liquid sulphur pours out in the water. It can be stretched in long fibres is unstable unstable. It easily solidifies.
protein → bjelančevina
Proteins are natural organic compounds of animal or herbal origin, essential in diet. They are natural polymers developed from a crowd of interconnecting monomers of amino acids, with relative molecular masses amounting up to a few million.
quantum numbers → kvantni brojevi
Quantum numbers describe the distance, shape and orientation of electronic orbitals.
solution → otopina
Solutions are homogeneous mixtures of clean substances. Solutions contain two or more substances mixed in a state of molecular dispersion. Component which is found in solution in greater amount than other components is called a solvent and other components are called dissolved substances. Solution can be unsaturated, saturated and oversaturated.
Hooke’s law → Hookeov zakon
Hooke’s law stating that the deformation of a body is proportional to the magnitude of the deforming force, provided that the body’s elastic limit (see elasticity) is not exceeded. If the elastic limit is not reached, the body will return to its original size once the force is removed. The law was discovered by English physicist Robert Hooke in 1676.
If a body on elastic spring is displaced from its equilibrium position (i.e. if the spring is stretched or compressed), a restitution force tries to return the body back in its equilibrium position. The magnitude of that force is proportional to the displacement of the body
Where F is the restitutional (elastic) force, x is the displacement of the body and k is the spring constant, which depends on dimensions, shape and material of the spring.
hybridization → hibridizacija
Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic orbitals are added together to generate new hybrid wave functions. The new orbitals which are formed are hybrids of the originals and have properties (shape, size and energy) that are somewhere in between.
Citing this page:
Generalic, Eni. "Tetrahedral molecular shape." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table