electrolytes → elektroliti
Electrolytes are substances which, when melted or dissolved in water, conduct electric current. By melting or dissolving they are dissociated into electrically charged particles (ions) which are able to conduct electric current. By passing of electric current the transfer of matter occurs. Positively charged particles (cations) travel towards the negative pole (the cathode) and negatively charged particles (the anions) travel towards the positive pole (the anode). Liquid metals, in which the conduction is by free electrons, are not usually regarded as electrolytes. Solid conductors of ions, as in the sodium-sulphur cell, are also known as electrolytes. Depending upon how it conducts electric current, matter can be divided into strong electrolytes, weak electrolytes and nonconductors.
electroplating → galvaniziranje
Electroplating (also called electrodeposition) is the deposition of a metallic coating onto an object by putting a negative charge onto the object and immersing it into a solution which contains a salt of the metal to be deposited. The metallic ions of the salt carry a positive charge and are attracted to the part. When they reach it, the negatively charged part provides the electrons to reduce the positively charged ions to metallic form.
Typically, a brass or nickel object is coated with a layer of silver by making use of electrolysis of a silver solution, using the object to be coated as the cathode. The anode consist of pure silver, and the cathode is the object to be plated. The electrolyte is a mixure of silver nitrate with potassium cyanide. The reactions are:
The cyanide ensures a low concentration of silver ions, a condition for providing the best plating results.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
entropy → entropija
Entropy (S) is a measure of the unavailability of a system’s energy to do work; in a closed system, an increase in entropy is accompanied by a decrease in energy availability. When a system undergoes a reversible change the entropy (S) changes by an amount equal to the energy (Q) transferred to the system by heat divided by the thermodynamic temperature (T) at which this occurs.
All real processes are to a certain extent irreversible changes and in any closed system an irreversible change is always accompanied by an increase in entropy.
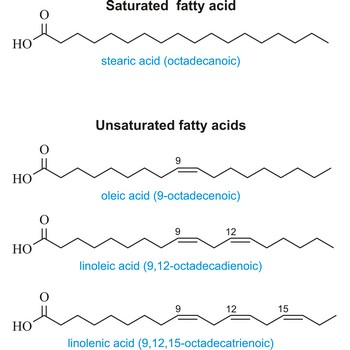
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
filter paper → filtar papir
Filter paper is a quantitative paper used for filtering and made of pure cellulose treated with hydrochloric and hydrofluoric acid. This kind of paper burns out practically without any remains (less than 0.0001 g ashes). Different types of paper are marked with numbers; qualitative bears marking 595 or 597 and quantitative 589 or 590. Dependable upon precipitate character, different types of filter paper are used:
- black band (5891) - 100 mL of fluid flows through it in 20 s to 30 s. It is used for filtering of gelatinous precipitates.
- white band (5892) - 100 mL of fluid flows through it in 40 s to 60 s. It is used for coarse crystalline precipitates filtration.
- blue band (5893) - 100 mL of fluid flows through it in 200 s to 400 s. It is used for fine crystalline precipitates.
millimetre of mercury → milimetar živina stupca
Millimetre of mercury (mmHg) is a non-SI unit of pressure, equal to 133.322 Pa. The name is generally considered interchangeable with torr.
Citing this page:
Generalic, Eni. "Tekuće agregatno stanje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table