glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
glass transition temperature → temperatura staklastog prijelaza
Glass transition temperature (Tg) is the temperature at which an amorphous polymer is transformed, in a reversible way, from a viscous or rubbery condition to a hard and relatively brittle one.
cryogenic fluid → kriogenski fluid
Cryogenic fluids are used for accessing low temperatures, usually below -150 °C. Cryogenic temperatures are achieved by the rapid evaporation of volatile liquids. The most common laboratory cryogenic fluids are liquid nitrogen (-196 °C). Nitrogen gas is colorless and odorless. The cloud formed when pouring liquid nitrogen is condensed water vapour from the air, not nitrogen gas.
Dewar flask → Dewarova posuda
Dewar flask or vacuum bottle is a container for storing hot or cold substances. It consists of two flasks, one placed inside the other, with a vacuum between. The vacuum prevents the conduction of heat from one flask to the other. For greater efficiency the flasks are silvered to reflect heat. The substance to be kept hot or cold, e.g., liquid air, is contained in the inner flask. The flask is named after British chemist and physicist Sir James Dewar (1842-1923). Dewar invented the Dewar flask in 1892 to aid him in his work with liquid gases. The common thermos bottle is an adaptation of the Dewar flask.
heat of reaction → toplina kemijske reakcije
Heat of reaction or enthalpy of reaction is the heat evolved or absorbed as a result of the complete chemical reaction of molar amounts of the reactants.
diffusion → difuzija
Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. The rate of diffusion is proportional to the concentration of the substance and increases with temperature. The theoretical principles are stated in Fick’s laws.
dissociation → disocijacija
Dissociation is the process by which a chemical combination breaks up into simpler constituents as a result of either added energy (dissociated by heat), or the effect of a solvent on a dissolved polar compound (electrolytic dissociation). It may occur in the gaseous, solid, or liquid state, or in a solution.
An example of dissociation is the reversible reaction of hydrogen iodide at high temperatures
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
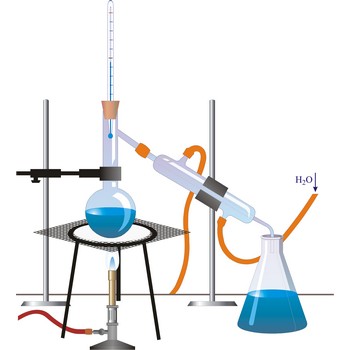
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
honey → med
Honey is a sweet, amber colored, viscous fluid produced by honeybees from the nectar of flowers. It is composed primarily of fructose (about 40 %), glucose (about 35 %), and water (up to 20 %). In addition, honey contains sucrose, maltose, trisaccharides, and small amounts of minerals, vitamins, and enzymes.
Citing this page:
Generalic, Eni. "Tekuće agregatno stanje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table