glucose → glukoza
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
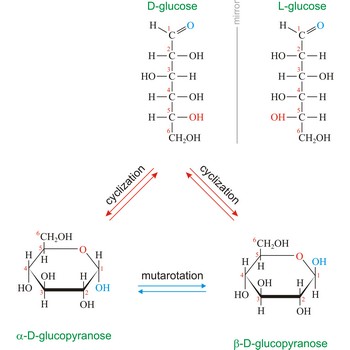
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
invert sugar → invertni šećer
Invert sugar is a mixture of equal parts of glucose and fructose resulting from the hydrolysis of sucrose (saccharose). The name stemming from the fact that it rotates of plane polarized light in the opposite direction of sucrose. Sucrose is dextrorotatory - it rotates polarized light clockwise ([α]D = +66.5°). Invert sugar rotates the plane of the polarized light counterclockwise ([α]D = -22°) due to the strongly levorotatory nature of fructose ([α]D = -92°).
Homemade artificial honey (invert sugar syrup): Dissolve two parts of household sugar (1 kg) with stirring in one part of water (0.5 kg) in a saucepan over low heat. Add 1 g of citric acid or the juice of one lemon to the mixture. Bring the ingredients to a slow boil. It can take anywhere between 15 minutes to 1 hour. The end result is sticky, golden syrup. Let it sit at room temperature until it is cool.
Gratzel solar cell → Gratzelova sunčeva ćelija
Grätzel solar cell is photoelectrochemical cell, developed by Michael Grätzel and collaborators, simulates some characteristics of the natural solar cell, which enables photosynthesis take place. In natural solar cell the chlorophyll molecules absorb light (most strongly in the red and blue parts of the spectrum, leaving the green light to be reflected). The absorbed energy is sufficient to knock an electron from the excited chlorophyll. In the further transport of electron, other molecules are involved, which take the electron away from chlorophyll. In Grätzel cell, the tasks of charge-carrier generation and transport are also assigned to different species.
His device consists of an array of nanometre-sized crystallites of the semiconductor titanium dioxide, welded together and coated with light-sensitive molecules that can transfer electrons to the semiconductor particles when they absorb photons. So, light-sensitive molecules play a role equivalent to chlorophyll in photosynthesis. In Grätzel cell, the light-sensitive molecule is a ruthenium ion bound to organic bipyridine molecules, which absorb light strongly in the visible range; titanium dioxide nanocrystals carry the received photoexcited electrons away from electron donors. On the other hand, a donor molecule must get back an electron, so that it can absorb another photon. So, this assembly is immersed in a liquid electrolyte containing molecular species (dissolved iodine molecules) that can pick up an electron from an electrode immersed in the solution and ferry it to the donor molecule. These cells can convert sunlight with efficiency of 10 % in direct sunlight and they are even more efficient in diffuse daylight.
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
magnesium → magnezij
Magnesium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word Magnesia, a district of Thessaly. It is lightweight, malleable, silvery-white metal. Burns in air with a brilliant white flame and reacts with water as temperature elevates. Can ignite in air. React violently with oxidants. Magnesium is found in large deposits in the form of magnesite, dolomite and other minerals. It is usually obtained by electrolysis of melted magnesium chloride (MgCl2) derived from brines, wells and sea water. Used in alloys to make airplanes, missiles and other uses for light metals. Have structural properties similar to aluminium.
mass-energy equivalence → ekvivalencija mase i energije
In the special theory of relativity Einstein demonstrated that neither mass nor energy were conserved separately, but that they could be traded one for the other and only the total "mass-energy" was conserved. The relationship between the mass and the energy is contained in what is probably the most famous equation in science,
Where m is the mass of the object and c is the velocity of light. Cockcroft and Walton (1932) are routinely credited with the first experimental verification of mass-energy equivalence.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
metre → metar
Metre (m) is the SI base unit of length.
The meter is the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 s.
This definition, adopted by the General Conference on Weights and Measure in October 1983, replaced the 1967 definition based on the krypton lamp.
microscope → mikroskop
Microscope is an instrument that produces enlarged images of small objects. The optical microscopes (light microscope) use visible light and a system of lenses to magnify images. Typical magnification of a light microscope is up to 1500× ("1500 times")with a theoretical resolution limit of around 200 nm. Instead of using light, electron microscopes transmit a beam of electrons through, or onto the surface of, a specimen. An electron beam has a much shorter wavelength than does light, and can reveal structures as small as 2 nm.
Citing this page:
Generalic, Eni. "Svjetlost." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table