body-centered orthorhombic lattice → prostorno centrirana ortorompska rešetka
Body-centered orthorhombic lattice (orthorhombic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
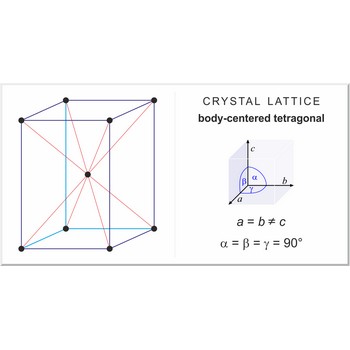
body-centered tetragonal lattice → prostorno centrirana tetragonska rešetka
Body-centered tetragonal lattice (tetragonal-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
caesium → cezij
Caesium was discovered by Robert Bunsen and Gustav Kirchhoff (Germany) in 1860. The origin of the name comes from the Latin word caesius meaning sky blue or heavenly blue. It is very soft, light grey, ductile metal. Reacts readily with oxygen. Reacts explosively with water. Caesium is found in pollucite [(Cs4Al4Si9O26)·H2O] and as trace in lepidolite. Used as a ’getter’ to remove air traces in vacuum and cathode-ray tubes. Also used in producing photoelectric devices and atomic clocks. Since it ionises readily, it is used as an ion rocket motor propellant.
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
electroorganic reaction → elektroorganske reakcije
Electroorganic reaction is an organic reaction produced in an electrolytic cell. Electroorganic reactions are used to synthesise compounds that are difficult to produce by conventional techniques. An example of an electroorganic reaction is Kolbe’s method of synthesising alkanes.
galvanostat → galvanostat
Galvanostat is an electronic instrument that controls the current through an electrochemical cell at a preset value, as long as the needed cell voltage and current do not exceed the compliance limits of the galvanostat.
lattice constants → konstante kristalne rešetke
Lattice constants are parameters specifying the dimensions of a unit cell in a crystal lattice, specifically the lengths of the cell edges and the angles between them.
carbohydrate → ugljikohidrat
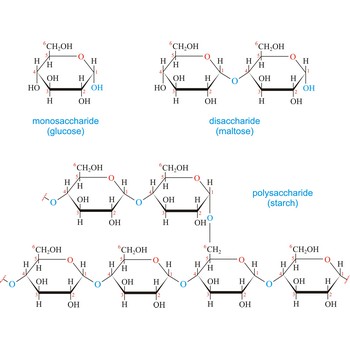
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
coulometer → kulometar
Coulometer is a type of electrolysis cell which is used for measuring the quantity of one element released during electrolysis.
Citing this page:
Generalic, Eni. "Sunčeva ćelija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table