Dalton, John → Dalton, John
Dalton, John (1766-1844) British chemist and physicist. In 1801 he formulated his law of partial pressures (Dalton’s law), but he is best remembered for Dalton’s atomic theory, which he announced in 1803. Dalton also studied colour blindness (a condition, once called Daltonism, that he shared with his brother).
chemical reaction equation → jednadžba kemijske reakcije
Chemical reactions are symbolically shown with chemical equations. On the left side of the equation we write formulas or substance symbols which enter the chemical reaction, reactants. On the right side formulas or substance symbols which emerge from the chemical reaction, products, are writen.
Each chemical reaction leads to an equilibrium which is moved more or less to one side (left or right). Because of that, in reversible reactions instead of = sign two opposite arrows are put
In order to write down certain chemical reaction equation all reactants and all products and their stechiometric proportions must be known. (See Chemical reaction balancing)
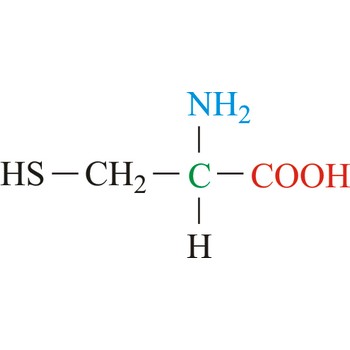
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
mercaptans → merkaptani
Mercaptans are a traditional term abandoned by IUPAC, synonymous with thiols, a sulfur-containing organic compound with the general formula R-SH where R is any radical. This term is still widely used.
Pauli exclusion principle → Paulijev princip zabrane
Pauli exclusion principle is the statement that two electrons in an atom cannot have identical all four quantum numbers. It was first formulated in 1925 by the Austrian-born Swiss physicst Wolfgang Ernst Pauli (1900-1958).
ether → eter
Ethers are organic compounds with a formula R-O-R, where R is not equal to H. They may be derived from alcohols by elimination of water, but the major method is catalytic hydration of olefins. They are volatile highly flammable compounds; when containing peroxides they can detonate on heating. The term ether is often used synonymously with diethyl ether.
Fajans’ rules → Fajansova pravila
Fajans’ rules, formulated by American chemist of Polish origin. Kazimierz Fajans (1887-1975), indicating the extent to which an ionic bond has covalent character caused by polarisation of the ions. Covalent character is more likely if:
1. the charge of the ions is high;
2. the positive ion is small or the negative ion is large;
3. the positive ion has an outer electron configuration that is not a noble- gas configuration.
Citing this page:
Generalic, Eni. "Strukturna formula." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table