strontium → stroncij
Strontium was discovered by Sir Humphry Davy (England) in 1808. Named after the village of Strontian in Scotland. It is soft, malleable, silvery-yellow metal. Combustible in air, will react with water. Exposed surfaces form protective oxide film. Metal ignites and burns readily. Strontium is found in minerals celestite and strontianite. Used in flares and fireworks for crimson colour. Strontium-90 is a long lived highly radioactive fallout product of atomic-bomb explosions.
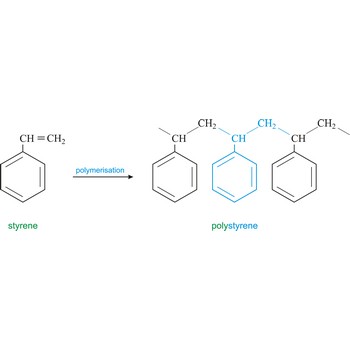
styrene → stiren
Styrene is an unsaturated hydrocarbon (C6H5OC2H3O) colourless, toxic liquid with a strong aromatic aroma. It is soluble in alcohol, ether, acetone, and carbon disulfide, but dissolves only slightly in water. It is used to make plastics such as polystyrene, ABS, styrene-butadiene rubber styrene-butadiene latex and unsaturated polyesters.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
sulfur → sumpor
Sulfur has been known since ancient times. The origin of the name comes from the Sanskrit word sulvere meaning sulphur; also from the Latin word sulphurium meaning sulphur. It is pale yellow, odourless, brittle solid, which is insoluble in water but soluble in carbon disulfide. Sulfur is found in pure form and in ores like cinnabar, galena, sphalerite and stibnite. Pure form is obtained from underground deposits by the Frasch process. Used in matches, gunpowder, medicines, rubber and pesticides, dyes and insecticides. Also for making sulfuric acid (H2SO4).
superfluid helium → superfluidni helij
Superfluidity in helium-4 was discovered in 1938 by the Soviet physicist Pyotr Leonidovich Kapitsa. Helium-4 exhibits superfluidity when it is cooled below 2.18 K (-270.97 C), which is called the lambda (λ) point. At these temperatures, helium-4 exhibits the characteristics of two distinct fluids, one of which appears to flow without friction. An extensive series of experiments showed that in this state of helium, called helium II (He II), there is an apparent enormous rise in heat conductivity, at an increase rate of about three million. Another unusual property of He II is its mobile, rapid flow through capillaries or over the rim of its containment vessel as a thin film that exhibits no measurable viscosity and appears unaffected by the forces of gravity or evaporation and condensation.
tantalum → tantal
Tantalum was discovered by Anders Ekeberg (Sweden) in 1802. The origin of the name comes from the Greek word Tantalos meaning father of Niobe in Greek mythology, (tantalum is closely related to niobium in the periodic table). It is rare, grey, heavy, hard but ductile, metal with a high melting point. Exposed surfaces form corrosion resistant oxide film. Attacked by HF and fused alkalis. Metal ignites in air. Tantalum always found with niobium. Chiefly occurs in the mineral tantalite. Often used as an economical substitute for platinum. Tantalum pentoxide is used in capacitors and in camera lenses to increase refracting power. It and its alloys are corrosion and wear resistant so it is used to make surgical and dental tools.
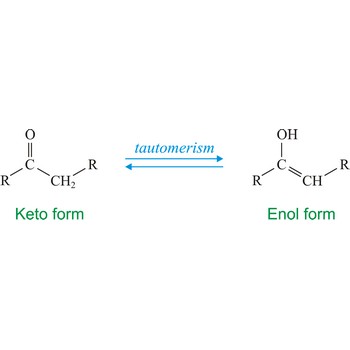
tautomerism → tautomerija
Tautomerism refers to equilibrium between two different structures of the same compound. Usually the tautomers differ in the point of attachment of a hydrogen atom. One of the most common examples of a tautomeric system is the equilibrium between a ketone (keto) and aldehyde (enol).
tellurium → telurij
Tellurium was discovered by Franz Joseph Muller von Reichstein (Romania) in 1782. The origin of the name comes from the Latin word tellus meaning earth. It is silvery-white, brittle semi-metal. Unreactive with water or HCl; dissolves in HNO3; burns in air or oxygen. Tellurium is obtained as a by-product of copper and lead refining. Used to improve the machining quality of copper and stainless steel products and to colour glass and ceramics. Also in thermoelectric devices. Some is used in the rubber industry and it is a basic ingredient in manufacturing blasting caps.
temporary hardness → prolazna tvrdoća
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. It can be removed by water reboiling, whereby white solid emerges calcium carbonate that is limescale.
terbium → terbij
Terbium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is soft, ductile, silvery-grey, rare earth metal. Oxidizes slowly in air. Reacts with cold water. Terbium is found with other rare earths in monazite sand. Other sources are xenotime and euxenite, both of which are oxide mixtures that can contain up to 1 % terbium. It is used in modest amounts in special lasers and solid-state devices.
Citing this page:
Generalic, Eni. "Slana voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table