electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
ether → eter
Ethers are organic compounds with a formula R-O-R, where R is not equal to H. They may be derived from alcohols by elimination of water, but the major method is catalytic hydration of olefins. They are volatile highly flammable compounds; when containing peroxides they can detonate on heating. The term ether is often used synonymously with diethyl ether.
europium → europij
Europium was discovered by Eugene Demarcay (France) in 1896. Named for the continent of Europe. It is soft, silvery-white metal. Extremely reactive with oxygen and water. Europium is obtained from monazite sand, which is a mixture of phosphates of calcium, thorium, cerium and most other rare earths. Used with yttrium oxide to make red phosphors for colour televisions.
Fahrenheit scale → Fahrenheitova skala
Fahrenheit scale is the temperature scale in which 212 degrees is the boiling point of water and 32 degrees is the freezing point of water. The scale was invented in 1714 by the German physicist G.D. Fahrenheit (1686-1736).
32 °F = 0 °C
212 °F = 100 °C
1 °F =(5/9) °C
T(°C) = (5/9)[T(°F) - 32]
T(°F) = (9/5)T(°C) + 32
primary alcohol → primarni alkohol
Primary alcohols are alcohols where the hydroxyl group is attached to a primary carbon atom. Thus, it has the general structure, RCH2OH, where R is a hydrogen atom or an alkyl group.
reactive metal → reaktivni metal
Reactive metals are metals that readily combine with oxygen at elevated temperatures to form very stable oxides, for example titanium, zirconium, and beryllium. Reactive metals may also become embrittled by the interstitial absorption of oxygen, hydrogen, and nitrogen.
relative humidity → relativna vlažnost
Relative humidity is the ratio of the partial pressure of water vapour in air to the saturation vapour pressure of water at the same temperature, expressed as a percentage.
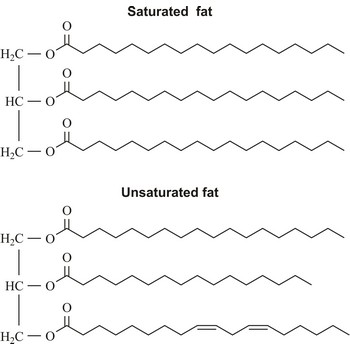
fat → mast
Fats are esters of glycerol and long chain carboxylic acids. Fats occur widely in plants and animals as a means of storing food energy, having twice the calorific value of carbohydrates. Fats derived from plants and fish generally have a greater proportion of unsaturated fatty acids than those from mammals. Fats may be either solid or liquid at room temperature, depending on their structure and composition. Unsaturated fats are liquid at room temperature.
Plant oils may be hardened by the addition of hydrogen atoms, converting double bonds to single bonds. This process is known as hydrogenation. Hydrogenated vegetable oils are often present in margarine and other processed foods.
Alkali hydrolysis of fat with sodium hydroxide it gives glycerol and soap (i.e. a mixture of the sodium salts of the fatty acids).
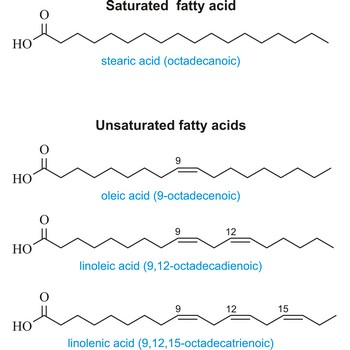
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
salinisation → salinizacija
Salinisation is the increase in salinity of fresh surface and ground water supplies.
Citing this page:
Generalic, Eni. "Slana voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table