system → sustav
System is the region under consideration, as distinguished from the rest of the universe (the environment). Systems may be separated from environments by boundaries that prevent the transfer of mass (a closed system), of heat (an adiabatic system), or of any energy (an isolated system). Systems that exchange mass with the environment are open systems.
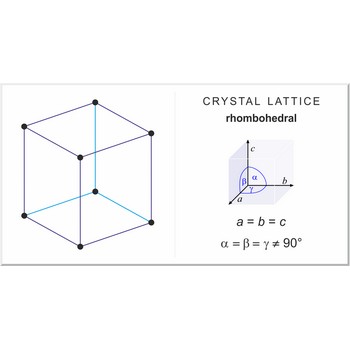
rhombohedral lattice → romboedarska rešetka
Rhombohedral (or trigonal) lattice has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b=c and interaxial angles α=β=γ≠90°.
Bravais lattice → Bravaisova rešetka
Bravais lattice is a set of points constructed by translating a single point in discrete steps by a set of basis vectors. The French crystallographer Auguste Bravais (1811-1863) established that in three-dimensional space only fourteen different lattices may be constructed. All crystalline materials recognised till now fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the bottom.
|
Crystal system
|
Bravais lattices
|
|||
|
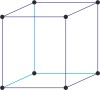
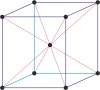
cubic a=b=c α=β=γ=90° |
 |
 |
 |
|
|
|
simple cubic
|
body-centered cubic
|
face-centered cubic
|
|
|
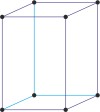
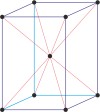
tetragonal a=b≠c α=β=γ=90° |
 |
 |
||
|
|
simple tetragonal
|
body-centered tetragonal
|
||
|
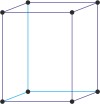
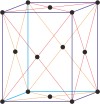
orthorhombic a≠b≠c α=β=γ=90° |
 |
 |
 |
 |
|
|
simple orthorhombic
|
base-centered orthorhombic
|
body-centered orthorhombic
|
face-centered orthorhombic
|
|
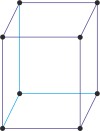
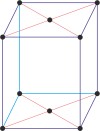
monoclinic a≠b≠c α=γ=90°≠β |
 |
 |
||
|
|
simple monoclinic
|
base-centered monoclinic
|
||
|
hexagonal a=b≠c α=β=90° γ=120° |
 |
|||
|
|
hexagonal
|
|||
|
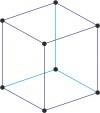
rhombohedral a=b=c α=β=γ≠90° |
 |
|||
|
|
rhombohedral
|
|||
|
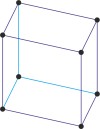
triclinic a≠b≠c α≠β≠γ≠90° |
 |
|||
|
triclinic
|
||||
crystallisation → kristalizacija
Crystallisation is process in which the melted substance from a saturated solution turns into solid substance (crystal).
crystallography → kristalografija
Crystallography is a science that studies structure, shapes, crystalline properties and laws of their creation.
fractional crystallisation → frakcijska kristalizacija
Fractional crystallisation is a method of separating a mixture of soluble solids by dissolving them in a suitable hot solvent and then lowering the temperature slowly. The least soluble component will crystallise out first, leaving the other components in the solution. By controlling the temperature, it is sometimes possible to remove each component in turn.
heat of crystallization → toplina kristalizacije
Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallises from a saturated solution of the same substance.
systematic error → sustavna pogreška
Systematic errors have an identifiable cause and affect the accuracy of results.
Citing this page:
Generalic, Eni. "Rhombohedral+crystal+system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table