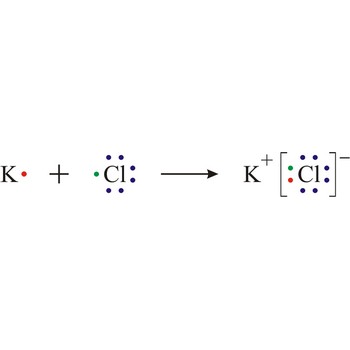polar solvent → polarno otapalo
Polar solvent is a liquid with polar molecules which dissolves polar compounds, because the charges on the endings of the solvent’s molecules attract the elements from ion crystals.
polariser → polarizator
Polariser is frequently a specially made prism from crystal of Iceland Spar (form of calcium carbonate), also known as Nicol’s prism. It can also be a small plate of tourmaline.
polymorphic transition → polimorfni prijelaz
Polymorphic transition is a reversible transition of a solid crystalline phase at a certain temperature and pressure to another phase of the same chemical composition with a different crystal structure. For examples, the transitions of quartz (SiO2) at 1 143 K to tridymite, and at 1 743 K to cristobalite.
Hesse’s law → Hessov zakon
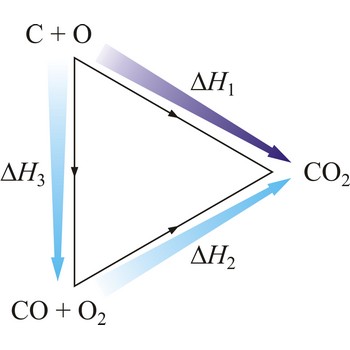
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
polyvalent molecule → polivalentna molekula
Polyvalent molecule is a molecule which having multiple binding sites. The antibodies of our immune system are one example.
potential of decomposition → napon razlaganja
Potential of decomposition of some system is the least potential which should be applied so that electrolysis should occur.
psychoactive drug → psihoaktivna droga
Psychoactive drugs are natural (mescaline) or synthetic substances (LSD) which take effect on central nervous system causing euphoria, and by lengthened use they also cause addiction, gradually destroying the nervous system.
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
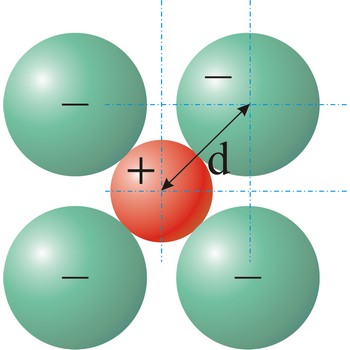
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
Citing this page:
Generalic, Eni. "Rhombohedral+crystal+system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table