polarogram → polarogram
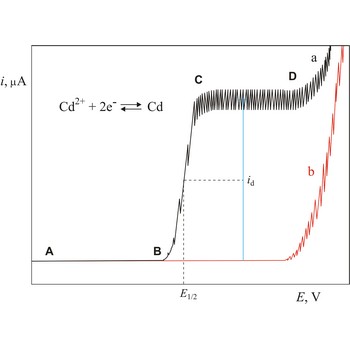
Polarogram is a graph of current versus potential in a polarographic analysis. The position of a polarographic wave in a polarogram along the x axis (E1/2) provides an identity of the substance while the magnitude of the limiting diffusion current (id) provides the concentration of this substance.
polarography → polarografija
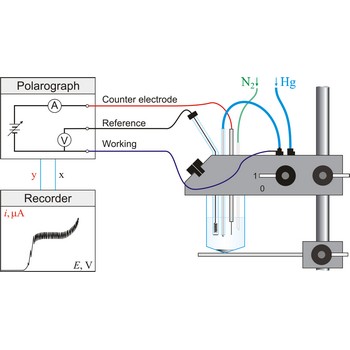
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
referent electrode → referentna elektroda
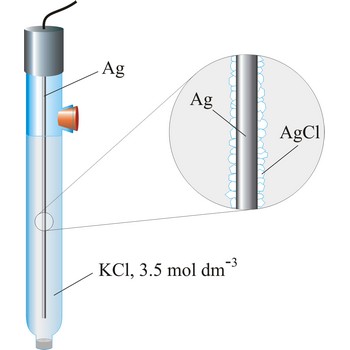
Referent electrode is an electrode whose potential is known and completely independent of analyte concentration. Mostly used referent electrodes are calomel and silver/silver chloride electrode.
Table: Dependence of referent electrodes potentials on KCl concentration
| Potential vs. SHE / V | |||||
| calomel electrode | Ag/AgCl electrode | ||||
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 | 0.212 | 0.209 |
| 20 | 0.3359 | 0.252 | 0.2479 | 0.208 | 0.204 |
| 25 | 0.3356 | 0.250 | 0.2444 | 0.205 | 0.199 |
| 30 | 0.3351 | 0.248 | 0.2411 | 0.201 | 0.194 |
| 35 | 0.3344 | 0.246 | 0.2376 | 0.197 | 0.189 |
salt bridge → solni most
Salt bridge is a permeable material soaked in a salt solution that allows ions to be transferred from one container to another. The salt solution remains unchanged during this transfer.
Schrodinger equation → Schrodingerova jednadžba
Schrödinger equation is the basic equation of wave mechanics which, for systems not dependent on time, takes the form:
where Ψ is the wavefunction, V is the potential energy expressed as a function of the spatial coordinates, E its total energy, ![]() 2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
supercritical carbon dioxide → superkritični ugljikov dioksid
Supercritical carbon dioxide (scCO2) is a powerful, cheap, non-toxic and environmental friendly solvent. When used at a supercritical state (over 74 bar and 31 °C), it achieves similar solvating power as its organic competitors, such as hydrocarbons and chlorinated solvents. Supercritical carbon dioxide is one of few solvents that can be unrestrictedly used for food processing.
Tafel plot → Tafelov dijagram
Tafel plot is the graph of the logarithm of the current density j against the overpotential η in electrochemistry in the high overpotential limit. An electrode when polarised frequently yields a current potential relationship over a region which can be approximated by:
where η is change in open circuit potential, i is the current density, B and i0 is constants. B is known as the Tafel Slope. If this behaviour is observed a plot of the semilogarithmic components is known as the Tafel line and the diagram is called the Tafel diagram.
Citing this page:
Generalic, Eni. "Redoks potencijal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table