non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
period → perioda
Periods are horizontal rows in the periodic table, each period begin with an alkali metal (one electron in the outermost principal quantum level) and ending with a noble gas (each having eight electrons in the outermost principal quantum level, except for helium, which is limited to two).
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
qualitative analysis → kvalitativna analiza
Qualitative analysis involves determining the nature of a pure unknown compound or the compounds present in a mixture. Qualitative inorganic analysis is used to separate and detect cations and anions in a sample substance. According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution.
radioactive indicator → radioaktivni indikator
By use of suitable radioactive isotopes biochemical processes can be observed in plants, animals and humans, by measuring radioactive radiation of radioactive indicator. Artificial radioactive isotopes have the same chemical properties as natural ones, which enable us to mark those natural isotopes with addition of artificial ones and in this way follow the path of those elements during a chemical reaction. One of the most important radioactive indicators is the radioactive carbon 14C.
radium → radij
Radium was discovered by Marie and Pierre Curie (France) in 1898. The origin of the name comes from the Latin word radius meaning ray. It is silvery-white radioactive metal. Reacts with oxygen and water. Highly radiotoxic. Carcinogen by inhalation, ingestion, or exposure. Radium is found in uranium ores at 1 part per 3 million parts uranium. Used in treating cancer because of the gamma rays it gives off.
seawater → more
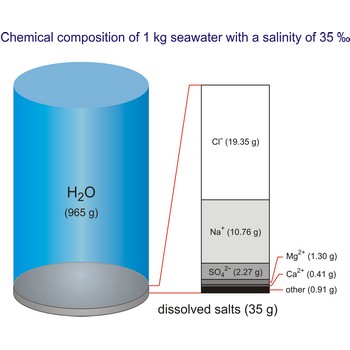
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
silicon → silicij
Silicon was discovered by Jöns Jacob Berzelius (Sweden) in 1824. The origin of the name comes from the Latin word silicis meaning flint. Amorphous form of silicon is brown powder; crystalline form has grey metallic appearance. Solid form unreactive with oxygen, water and most acids. Dissolves in hot alkali. Silica dust is a moderately toxic acute irritant. Silicon makes up major portion of clay, granite, quartz and sand. Commercial production depends on a reaction between sand (SiO2) and carbon at a temperature of around 2200 °C. Used in glass as silicon dioxide (SiO2). Silicon carbide (SiC) is one of the hardest substances known and used in polishing. Also the crystalline form is used in semiconductors.
standard deviation → standardna devijacija
Standard deviation (σ) is a measure of the dispersion of a set of data from its mean. Standard deviation is a statistical term that measures the amount of variability or dispersion around an average
Suppose there are many measurements of a quantity presumed to be similar, like the size of peas in a pod. If the number of readings for each size were plotted, a bell-shaped curve would probably result, with a few small and large peas and most clustered around the average size. Around two-thirds of all measurements fall in the range spanned by the standard deviation, a measure of the spread.
Citing this page:
Generalic, Eni. "Rasprostranjenost elemenata." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table