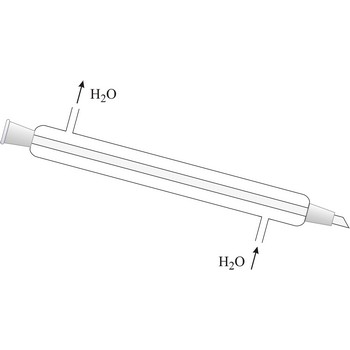
reflux condenser → povratno hladilo
Reflux condenser is used for repeated transformation of vapour in liquid in order to prevent the loss due to evaporation.
Allihn condenser → Allihnovo hladilo
Allihn condenser or bulb condenser consists of an outer water jacket and the inner glass tube with a series of spherical bubbles to maximize the thermal contact with the cooling water. It is named after its inventor, the German chemist Felix Richard Allihn (1854-1915).
Liebig condenser → Liebigovo hladilo
Liebig condenser is used for condensing of vapours that pass trough the centre tube. It is cooled with water that passes in the outer tube (shell around the centre tube) in the opposite direction than the one of hot vapour. Though named after the German chemist Justus von Liebig (1803-1873), he cannot be given credit for having invented it because it had already been in use for some time before him.
reverse reaction → povratna reakcija
Reverse reaction is a reaction in backward directions, reaction (in the reversible reaction) in which original reactants emerge again from a products. It goes from right to left.
chemical balance → kemijska ravnoteža
Chemical balance is a degree of reversible reaction in a closed system, when the forward and backward reaction happen at same rates and their effects annul each other, while the concentration of reactants and products stays the same.
cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
harmonic motion → harmoničko gibanje
Harmonic motion is caused by restoring force, acting on a body that is displaced from its equilibrium position. This force tries to put the body back in equilibrium. Usual examples are the motion of a body attached to elastic spring (see: Hooke’s law) and the motion of mathematical pendulum. The body undergoes periodic motion around the equilibrium point.
Hooke’s law → Hookeov zakon
Hooke’s law stating that the deformation of a body is proportional to the magnitude of the deforming force, provided that the body’s elastic limit (see elasticity) is not exceeded. If the elastic limit is not reached, the body will return to its original size once the force is removed. The law was discovered by English physicist Robert Hooke in 1676.
If a body on elastic spring is displaced from its equilibrium position (i.e. if the spring is stretched or compressed), a restitution force tries to return the body back in its equilibrium position. The magnitude of that force is proportional to the displacement of the body
Where F is the restitutional (elastic) force, x is the displacement of the body and k is the spring constant, which depends on dimensions, shape and material of the spring.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
Citing this page:
Generalic, Eni. "Povratno hladilo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table