active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
adenosine triphosphate → adenozin trifosfat
Adenosine triphosphate (ATP) is nucleotide that is of fundamental importance as a carrier of chemical energy in all living organisms. It consists of adenin linked to D-ribose).
alanine → alanin
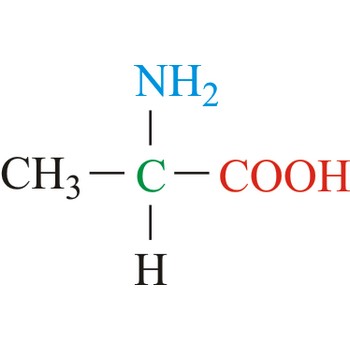
Alanine is hydrophobic amino acids with aliphatic side chain. It is the second simplest amino acid, but used the most in proteins. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained directly through the diet.
- Abbreviations: Ala, A
- IUPAC name: 2-aminopropanoic acid
- Molecular formula: C3H7NO2
- Molecular weight: 89.09 g/mol
Allihn condenser → Allihnovo hladilo
Allihn condenser or bulb condenser consists of an outer water jacket and the inner glass tube with a series of spherical bubbles to maximize the thermal contact with the cooling water. It is named after its inventor, the German chemist Felix Richard Allihn (1854-1915).
allotrope → alotrop
Allotropes are the elements which exist in two or more different forms in the same physical state. Allotropes generally differ in physical properties and may also differ in chemical activity.
Diamond, graphite and fullerenes are three allotropes of the element carbon. Graphite is a soft, black, slippery substance; by contrast, diamond is one of the hardest substances known. The different properties of the allotropes arise from their chemical structures. Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Graphite crystallizes in the hexagonal system. In the fullerenes, the carbon atoms taking the form of a hollow sphere, ellipsoid, or tube.
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C.
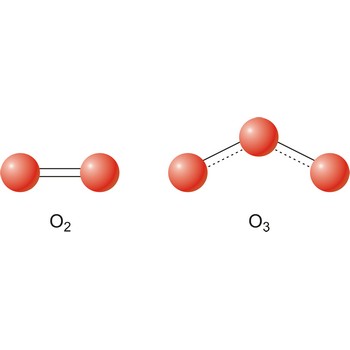
The term allotropes may also be used to refer to the molecular forms of an element. Ozone is a chemically active triatomic allotrope of the element oxygen.
allotropic modification → alotropska modifikacija
Different substances of the same elementary system are called allotropes or allotropic modifications. In the case of oxygen, there are two allotropic modifications: "normal" dioxygen (O2) and trioxygen (O3) or ozone.
analytical balance → analitička vaga
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
anomer → anomer
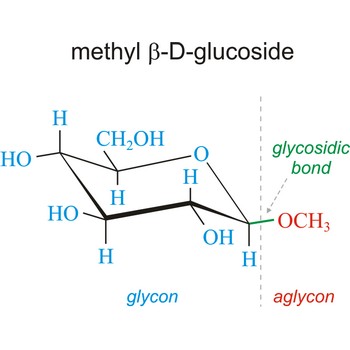
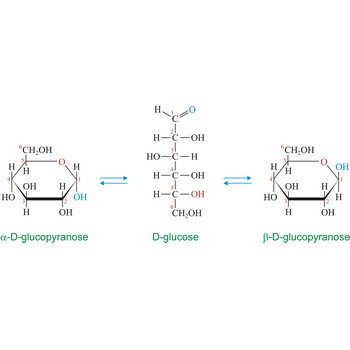
Anomers are diastereoisomers of cyclic forms of sugars or similar molecules differing in the configuration at the anomeric carbon (C-1 atom of an aldose or the C-2 atom of a 2-ketose). The cyclic forms of carbohydrates can exist in two forms, α- and β- based on the position of the substituent at the anomeric center. Anomer are designated α if the configuration at the anomeric carbon is the same as that at the reference asymmetric carbon in a Fischer projection. If the configuration differs the anomer is designated β. For example, α-D-glucopyranose and β-D-glucopyranose, the two cyclic forms of glucose, are anomers.
Citing this page:
Generalic, Eni. "Periodni_sustav_elemenata." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table