analytical balance → analitička vaga
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
anomer → anomer
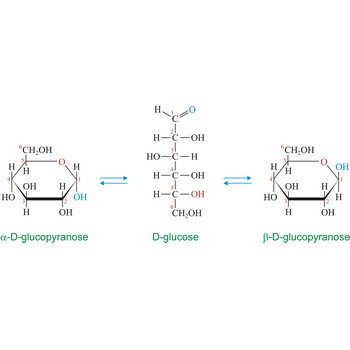
Anomers are diastereoisomers of cyclic forms of sugars or similar molecules differing in the configuration at the anomeric carbon (C-1 atom of an aldose or the C-2 atom of a 2-ketose). The cyclic forms of carbohydrates can exist in two forms, α- and β- based on the position of the substituent at the anomeric center. Anomer are designated α if the configuration at the anomeric carbon is the same as that at the reference asymmetric carbon in a Fischer projection. If the configuration differs the anomer is designated β. For example, α-D-glucopyranose and β-D-glucopyranose, the two cyclic forms of glucose, are anomers.
arginine → arginin
Arginine is an electrically charged amino acids with basic side chains. It is one of the least frequent amino acids. As a group the charged amino acids are important for making proteins soluble. These residues are generally located on the surface of the protein. Arginine is well designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates. As a cation, arginine, as well as lysine, plays a role in maintaining the overall charge balance of a protein. Although arginine is considered an essential amino acid (it must be obtained through the diet), this is true only during the juvenile period in humans.
- Abbreviations: Arg, R
- IUPAC name: 2-amino-5-(diaminomethylideneamino)pentanoic acid
- Molecular formula: C6H14N4O2
- Molecular weight: 174.20 g/mol
asparagine → asparagin
Asparagine is neutral amino acids with polar side chains. The polar amino acids are an important class of amino acids since they provide many of the functional groups found in proteins. Asparagine is a common site for attachment of carbohydrates in glycoproteins. In general this is not very reactive residues. Asparagine is amide derivative of aspartic acid. Asparagine is not essential for humans, which means that it can be synthesized from central metabolic pathway intermediates and is not required in the diet.
- Abbreviations: Asn, N
- IUPAC name: 2,4-diamino-4-oxobutanoic acid
- Molecular formula: C4H8N2O3
- Molecular weight: 132.12 g/mol
aspartic acid → asparaginska kiselina
Aspartic acid is an electrically charged amino acids with acidic side chains. As a group the charged amino acids are relatively abundant and are generally located on the surface of the protein. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Aspartic acid (sometimes referred to as asparate depending on pH) is non-essential in mammals, being produced from oxaloacetate by transamination.
- Abbreviations: Asp, D
- IUPAC name: 2-aminobutanedioic acid
- Molecular formula: C4H7NO4
- Molecular weight: 133.10 g/mol
atmosphere → atmosfera
1. Atmosphere is the column of air which is extending several hundred kilometers above the surface the Earth's surface. The density of this air decreases as you proceed up from the surface. The air in the atmosphere consists of 78 % nitrogen, 21 % oxygen, and 0.9 % argon. The remaining 0.1 % of the atmosphere consists of ozone, water vapor, carbon dioxide, methane, helium, and neon. The atmosphere is divided into different regions. The lowest two layers are the troposphere (the layer closest to the earth) and the stratosphere respectively. These two layers contain more than 99 % of the atmospheric molecules.
2. Standard atmosphere (atm) is an obsolete pressure and stress unit which should be discontinued. It is unit of pressure equal to the air pressure measured at mean sea level.
1 atm = 101 325 Pa
Technical atmosphere (at) is an obsolete MKpS pressure and sttress derived unit.
1 at = 98 066.5 Pa
1 atm = 1.033 227 453 at
atom → atom
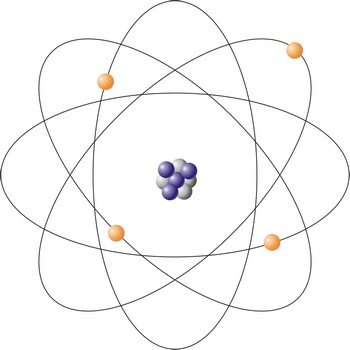
Atom is an atom is the smallest particle of an element that retains the chemical properties of the element. Rutherford-Bohr’s model represents the atom as a positively charged core of a size around 10-14 m composed of protons (positive particles) and neutrons (neutral particles) around which negatively charged electrons circle. The number of protons and electrons are equal, so the atom is an electrically a neutral particle. Diameter of the atom is about 10-10 m.
atom radius → radijus atoma
Atoms and molecules have no strict boundaries. The volume of a free atom is usually defined as that volume that contains 90 % of electron cloud. The radius of an atom represents half of interatom distance of two identical atoms which are in touch but are not interconnected either by a covalent or an ionic bond, but with a very weak van der Waals’s bond.
automatic burette → automatska bireta
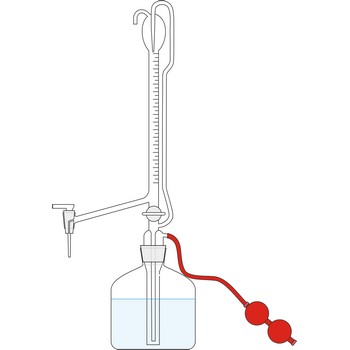
Automatic burette is used for series of tests. It is connected with a bottle which contains the titration solution. The air is pumped into the bottle by a small rubber air pump, created the pressure in the bottle which the rises the solution to the top of burette. When the the burette is full, the valve is released, the pressure in the bottle falls and the burette automatically sets itself to zero. Work with automatic burettes is by far faster and the consumption of standard solution is smaller.
bag filter → vrećasti filtar
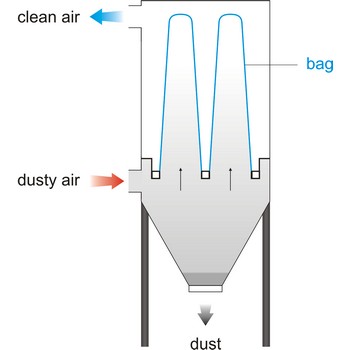
Bag filter is a unit within a mechanical system that bellows in a bag form when air flows through, cleaning the air by collecting particles of foreign matter. This system of filtering is rated the most efficient- in a range of 92 % to 95 %. Vacuum cleaner have a cloth filter bag.
Bag filter system is an economical method of liquid filtration consisting of the pressure vessel, restrainer basket and micron rated filter bag. Liquid flow is from the inside to the outside of the bag - dirt is trapped inside the bag.
Citing this page:
Generalic, Eni. "Perioda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table