proton → proton
Proton is a stable elementary particle of unit positive charge and spin 1/2. Protons and neutrons, which are collectively called nucleons, are the constituents of the nucleus.
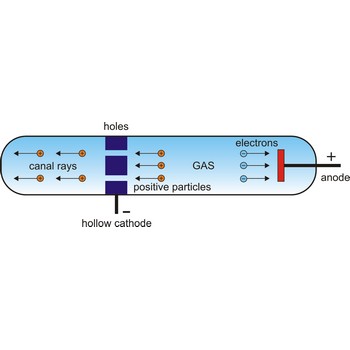
In 1886, German physicist Eugene Goldstein (1850-1930) discovered positive particles by using a modified Crookes tube with holes in the cathode in an evacuated tube. When cathode rays were given off in one direction toward the anode, other rays found their way through the holes in the cathode and sped off in the opposite direction. Since these other rays traveled in the direction opposite to the negatively charged cathode rays, it seemed that they must be composed of positively charged particles. Rutherford suggested that this fundamental positive particle be called the proton.
qualitative analysis → kvalitativna analiza
Qualitative analysis involves determining the nature of a pure unknown compound or the compounds present in a mixture. Qualitative inorganic analysis is used to separate and detect cations and anions in a sample substance. According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution.
radioactive series → radioaktivni niz
Radioactive series is a sequence of nuclides formed by successive radioactive decays until a stable decay product, the end product, is formed. A famous example of a radioactive series is the decay of uranium, which through a series of steps decays into stable lead.
rare earth elements → elementi rijetkih zemalja
Rare earth elements (metals) are the elements scandium (Sc), yttrium (Y), and the lanthanides (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). These elements got their name from the fact that chemists first isolated them in their oxide forms. These oxides somewhat resemble calcium, magnesium and aluminium oxides, sometimes called common earths. Do you want to know more?
redox potential → redoks potencijal
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
reflux condenser → povratno hladilo
Reflux condenser is used for repeated transformation of vapour in liquid in order to prevent the loss due to evaporation.
refractometer → refraktometar
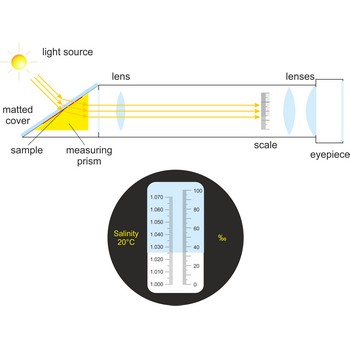
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
resonance → rezonancija
Resonance is a stabilising quality of certain molecules that can be represented by considering the electron distribution in an ion or molecule as a composite of two or more forms, in those cases where a single form is an inadequate representation; for example, benzene and the carbonate ion. A various canonical structures can be drawn to show how electron delocalisation will explain the discrepancy, the difference in electron density
retardation factor → faktor zaostajanja
Retardation factor, RF, (in planar chromatography) is a ratio of the distance travelled by the centre of the spot to the distance simultaneously travelled by the mobile phase:
The RF value is characteristic for any given compound on the same stationary phase using the same mobile phase for development of the plates. Hence, known RF values can be compared to those of unknown substances to aid in their identifications.
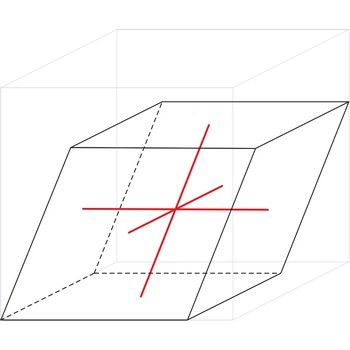
rhombohedral crystal system → romboedarski kristalni sustav
Rhombohedral crystal system is also known as the trigonal system. The crystallographic axes used in this system are of equal length. None of the axes are perpendicular to any other axis.
a = b = c
α= β = γ ≠ 90°
Citing this page:
Generalic, Eni. "Perioda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table