gas → plin
Gas is a state of matter, in which the mollecules move freely and consequently the entire mass tends to expand indefinitely, occupying the total volume of any vessel into which it is introduced. Gases follow, within considerable degree of fidelity, certain laws relating their conditions of pressure, volume and temperature. Gases mix freely with each other, and they can be liquefied through compression or temperature reduction.
gas washing bottle → boca ispiralica
Gas washing bottle or Drechsel bottle provide an inexpensive but effective method for washing or drying gases. The gas enters the bottle through the top of the central vertical tube, the lower end of which is below the surface of the washing medium. To maximize surface area contact of the gas to the liquid, a gas stream is slowly blown into the vessel through the fritted glass tip so that it breaks up the gas into many tiny bubbles. After bubbling through the medium, the gas rises to the top and exits through the side tube. It is named after the German chemist Edmund Drechsel (1843-1897).
glass electrode → staklena elektroda
Glass electrode is a hydrogen-ion responsive electrode usually consisting of a bulb, or other suitable form, of special glass attached to a stem of high resistance glass complete with internal reference electrode and internal filling solution system. Glass electrode is also available for the measurement of sodium ions.
The glass electrode, which consists of a thin wall glass bulb, has an extremely high electrical resistance. The membrane of a typical glass electrode (with a thickness of 0.03 mm to 0.1 mm) has an electrical resistance of 30 MΩ to 600 MΩ. The surface of a glass membrane must be hydrated before it will function as a pH electrode. When a glass surface is immersed in an aqueous solution then a thin solvated layer (gel layer) is formed on the glass surface in which the glass structure is softer. This applies to both the outside and inside of the glass membrane.
The simplest explanation for the working of the thin glass electrode is that the glass acts as a weak acid (Glass-H).
The hydrogen ion activity of the internal solution is held constant. When a solution of different pH from the inside comes in contact with the outside of the glass membrane, the glass is either deprotonated or protonated relative to the inside of the glass. The difference in pH between solutions inside and outside the thin glass membrane creates electromotive force in proportion to this difference in pH.
global warming → globalno zatopljenje
Global warming or greenhouse effect is an effect occurring in the atmosphere because of the presence of certain gases (greenhouse gases) that absorb infrared radiation. Light and ultraviolet radiation from the sun is able to penetrate the atmosphere and warm the Earth’s surface. This energy is re-radiated as infrared radiation which because of its longer wavelength, is absorbed by such substances as carbon dioxide. The overall effect is that the average temperature of the Earth and its atmosphere is increasing (so-called global Warming). The effect is similar to that occurring in a greenhouse, where light and long-wavelength ultraviolet radiation can pass through the glass into greenhouse but the infrared radiation is absorbed by the glass and part of it is re-radiated into the greenhouse.
The greenhouse effect is seen as a major environmental hazard. Average increases in temperature could change weather patterns and agricultural output. It might also lead to melting of the polar ice caps and a corresponding rise in sea level. Carbon dioxide, from fossil-fuel power stations and car exhausts, is the main greenhouse gas. Other contributory pollutants are nitrogen oxides, ozone, methane, and chloroflourocarbons.
glutamic acid → glutaminska kiselina
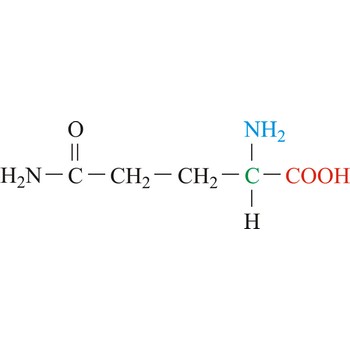
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
glucose → glukoza
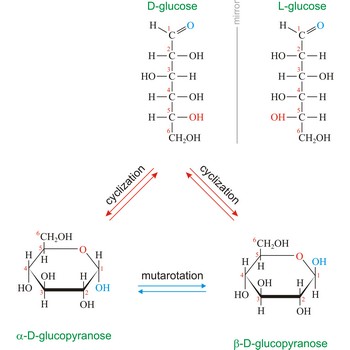
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
glutamine → glutamin
Glutamine is neutral amino acids with polar side chains. It serves as an important carrier of ammonia and contributes it to the formation of urea and purines. Glutamine is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders. It is synthesized by the enzyme glutamine synthetase from glutamate and ammonia.
- Abbreviations: Gln, Q
- IUPAC name: 2,5-diamino-5-oxopentanoic acid
- Molecular formula: C5H10N2O3
- Molecular weight: 146.14 g/mol
glycine → glicin
Glycine is the smallest amino acid and is unique because it lacks a side chain. This gives it more conformational freedom than any other amino acid. Glycine is often found in turns and loops where other amino acids would be sterically unacceptable. Although it is formally nonpolar, it’s very small side chain makes no real contribution to hydrophobic interactions. Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine.
- Abbreviations: Gly, G
- IUPAC name: 2-aminoacetic acid
- Molecular formula: C2H5NO2
- Molecular weight: 75.07 g/mol
glycogen → glikogen
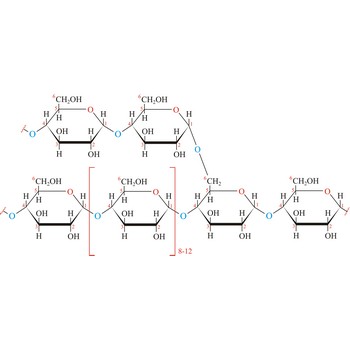
Glycogen (animal starch) is a polysaccharide that serves the same energy storage function in animals that starch serves in plants. Dietary carbohydrates not needed for immediate energy are converted by the body to glycogen for long term storage (principally in muscle and liver cells). Like amylopectin found in starch, glycogen is a polymer of α(1→4)-linked subunits of glucose, with α(1→6)-linked branches. Glycogen molecules are larger than those of amylopectin (up to 100 000 glucose units) and contain even more branches. Branch points occur about every 10 residues in glycogen and about every 25 residues in amylopectin. The branching also creates lots of ends for enzyme attack and provides for rapid release of glucose when it is needed.
Citing this page:
Generalic, Eni. "Perioda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table