ebullioscopic constant → ebulioskopska konstanta
Ebullioscopic constant (Eb) is the constant that expresses the amount by which the boiling point Tb of a solvent is raised by a nondissociating solute, through the relation
where b is the molality of the solute.
electroanalytical chemistry → elektroanalitička kemija
Electroanalytical chemistry chemistry is the application of electrochemical cells and electrochemical techniques for chemical analysis. The analyte is dissolved in the electrolyte of the cell, and one can perform either qualitative analysis (determination of the type of constituents present) or quantitative analysis (determination of the amount of a given constituent).
dioxin → dioksin
Dioxin is a general term that describes a group of hundreds of chemicals that are highly persistent in the environment. The most toxic compound is 2,3,7,8-tetrachlorodibenzo-p-dioxin or TCDD. The toxicity of other dioxins and chemicals like PCBs that act like dioxin are measured in relation to TCDD. Dioxin is formed as an unintentional by-product of many industrial processes involving chlorine such as waste incineration, chemical and pesticide manufacturing and pulp and paper bleaching. Dioxin was the primary toxic component of Agent Orange, found at Love Canal in Niagara Falls, NY and was the basis for evacuations at Times Beach, MO and Seveso, Italy.
Dioxin is formed by burning chlorine-based chemical compounds with hydrocarbons. The major source of dioxin in the environment comes from waste-burning incinerators of various sorts and also from backyard burn-barrels. Dioxin pollution is also affiliated with paper mills which use chlorine bleaching in their process, with the production of Polyvinyl Chloride (PVC) plastics, and with the production of certain chlorinated chemicals (like many pesticides).
dipole moment → dipolni moment
Electric dipole moment (μ) is a product of the positive charge and the distance between the charges. Dipole moments are often stated in debyes; The SI unit is the coulomb metre. In a diatomic molecule, such as HCl, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom (in the case of HCl, the electrons are attracted towards the more electronegative chlorine atom). In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4) there is no overall dipole moment, although the individual C-F bonds are polar.
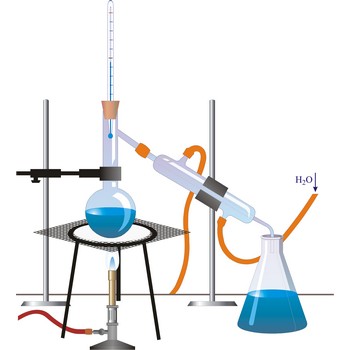
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
equivalent → ekvivalent
Equivalent (eq) is a unit for describing the amount of a chemical species. In contrast to the mole, the amount of a substance contained in one equivalent can vary from reaction to reaction.
distilled water → destilirana voda
Distilled water is water purified by distillation so as to free it from dissolved salts and other compounds. Distilled water in equilibrium with the carbon dioxide in the air has conductivity of about 0.8×10-6 S cm-1. Repeated distillation in vacuum can bring conductivity down to 0.043×10-6 S cm-1 at 18 °C. The limiting conductivity is due to self ionisation
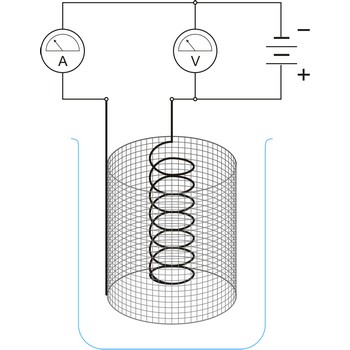
electrochemical cell → elektrokemijski članak
Electrochemical cell is a device that converts chemical energy into electrical energy or vice versa when a chemical reaction is occurring in the cell. It consist of two electronically conducting phases (e.g., solid or liquid metals, semiconductors, etc) connected by an ionically conducting phase (e.g. aqueous or non-aqueous solution, molten salt, ionically conducting solid). As an electric current passes, it must change from electronic current to ionic current and back to electronic current. These changes of conduction mode are always accompanied by oxidation/reduction reactions.
An essential feature of the electrochemical cell is that the simultaneously occurring oxidation-reduction reactions are spatially separated. E.g., in a spontaneous chemical reaction during the oxidation of hydrogen by oxygen to water, electrons are passed directly from the hydrogen to the oxygen.
In contrast, in the spontaneous electrochemical reaction in a galvanic cell the hydrogen is oxidised at the anode by transferring electrons to the anode and the oxygen is reduced at the cathode by accepting electrons from the cathode. The ions produced in the electrode reactions, in this case positive hydrogen ions and the negative hydroxyl (OH-) ions, will recombine in the solution to form the final product of the reaction: water. During this process the electrons are conducted from the anode to the cathode through an outside electric circuit where the electric current can drive a motor, light a light bulb, etc. The reaction can also be reversed: water can be decomposed into hydrogen and oxygen by the application of electrical power in an electrolytic cell.
electrogravimetry → electrogravimetrija
Electrogravimetry is an electroanalytical technique in which the substance to be determined (usually a metal) is deposited out on an electrode which is weighed before and after the experiment. The potential of the electrode must be carefully chosen to ensure that only the metal do be determined will deposit.
Citing this page:
Generalic, Eni. "Optički aktivna tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table