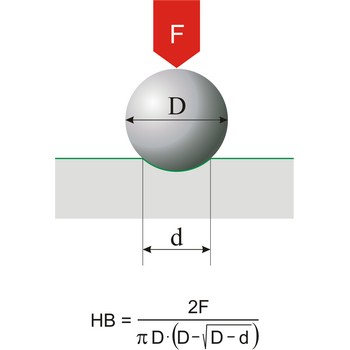
Brinell hardness → Brinellova tvrdoća
Brinell hardness is a scale for measuring the hardness of metals introduced around 1900 by Swedish metallurgist Johan Brinell (1849-1925). A small chromium steel ball is pressed into the surface of the metal by a load of known weight. The loading force is in the range of 300 N to 30 000 N. The ratio of the mass of the load in kilograms to the area of the depression formed in square millimetres is the Brinell Hardness Number.
bronze → bronca
Bronze is an alloy made primarily of copper and tin. It may contain as much as 25 % tin. Bronzes with 10 % or more tin are harder, stronger, and resistant to corrosion. As bronze weathers, a brown or green film forms on the surface. This film inhibits corrosion. Silicon or aluminium is often added to bronze to improve resistance to corrosion. Phosphorus, lead, zinc, and other metals may be added for special purposes. The alloy is hard and easily cast and is extensively used in bearings, valves and other machine parts.
Bronze was one of the first alloys developed by ancient metal workers. The Bronze Age occurred in Europe around 2200 to 700 BC. Bronze was used for weapons such as spearheads, swords, and knives. Since ancient times, bronze has been the most popular metal for casting statues and other art objects.

The term bronze has been adopted commercially for many copper-rich alloys that contain little or no tin but are similar in colour to bronze, including aluminium bronze, manganese bronze, and silicon bronze. Aluminium bronze is used to make tools and, because it will not spark when struck. Manganese bronze is actually a brass that contains manganese. It is often used to make ship propellers because it is strong and resists corrosion by sea water.
Bunsen burner → Bunsenov plamenik
Bunsen burner is a standard source of heat in the laboratory. German chemist Roberts Bunsen (1811-1899) improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. The Bunsen burner has a vertical metal tube through which a fine jet of fuel gas is directed. Air is drawn in through airholes near the base of the tube and the mixture is ignited and burns at the tube’s upper opening. The flow of this air is controlled by an adjustable collar on the side of the metal tube. When the whole is closed a yellow safety flame is displayed. Where as when the whole is open it displays a power dull blue flame with a faint blue outer flame with a vibrant blue core used u for combustion and hearting. The flame can reach temperatures of 1 500 °C.
elementary substance → elementarna tvar
Elementary substance is a simple and pure substance which can not be, by chemistry methods, decomposed further into simpler substances.
Faraday cage → Faradayev kavez
Faraday cage is a container giving protection from electrical fields: an assembly of conducting material, for example, metal mesh or grid, placed around electrical equipment to protect it from external electrical fields. Faraday cages are named after the English scientist Michael Faraday (1791-1867).
Bunsen, Robert Wilhem → Bunsen, Robert Wilhem
Robert Wilhem Bunsen (1811-1899) is a German chemist who held professorships at Kassel, Marburg and Heidelberg. His early researches on organometallic compound of arsenic cost him an eye in an explosion. Bunsen's most important work was in developing several techniques used in separating, identifying, and measuring various chemical substances. He also improvement chemical battery for use in isolating quantities of pure metals - Bunsen battery.
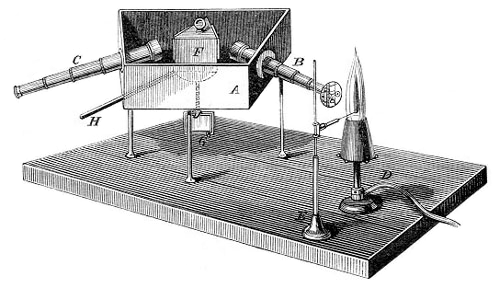
The essential piece of laboratory equipment that has immortalized the name of Bunsen was not invented by him. Bunsen improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the German physicist Gustav Kirchoff and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
caesium → cezij
Caesium was discovered by Robert Bunsen and Gustav Kirchhoff (Germany) in 1860. The origin of the name comes from the Latin word caesius meaning sky blue or heavenly blue. It is very soft, light grey, ductile metal. Reacts readily with oxygen. Reacts explosively with water. Caesium is found in pollucite [(Cs4Al4Si9O26)·H2O] and as trace in lepidolite. Used as a ’getter’ to remove air traces in vacuum and cathode-ray tubes. Also used in producing photoelectric devices and atomic clocks. Since it ionises readily, it is used as an ion rocket motor propellant.
Citing this page:
Generalic, Eni. "Meta položaj." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table