lactic acid → mliječna kiselina
Lactic acid is an acid produced as a result of anaerobic respiration in muscles and red blood cells, i.e. when glycogen is used as an energy source for respiration rather than oxygen. After production, it is converted back to glycogen in the liver. The build up of large amounts of lactic acid in the blood can lead to stress and toxic effects. High levels are usually a result of sustained, excessive exercise.
nucleic acid → nukleinska kiselina
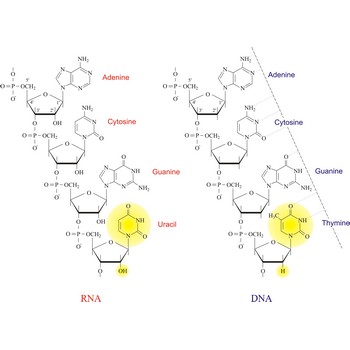
Nucleic acids are a complex, high-molecular-weight biochemical macromolecules composed of nucleotide chains that convey genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Each nucleic acid chain is composed of subunits called nucleotides, each containing a sugar, a phosphate group, and nitrogenous base. DNA was first discovered in 1869 by the Swiss biochemist Friedrich Miescher (1844-1895).
Both DNA and RNA contain the two major purine bases adenine (A) and guanine (G) and one of the major pyrimidines, cytosine (C). Of the other two pyrimidines, thymine (T) is found in DNA and uracil (U) is found in RNA. There are two major pentoses in nucleic acids:2'-deoxy-D-ribose in DNA and D-ribose in RNA.
Nucleotides are linked together in both DNA and RNA in a polymeric fashion via covalent bonds. These bonds exist through phosphate-group bridges in which the 5' hydroxyl group of one nucleotide unit is joined to the 3' hydroxyl group of the next nucleotide. RNA is usually a single-stranded molecule, whereas DNA is usually double-stranded.
ribonucleic acid → ribonukleinska kiselina
Ribonucleic acid is a complex organic compound in living cells that is concerned with protein synthesis. Plays an intermediary role in converting the information contained in DNA into proteins. RNA carries the genetic information from DNA to those parts of the cell where proteins are made. Some viruses store their genetic information as RNA not as DNA.
Ribonucleic acid is a similar molecule to DNA but with a slightly different structure.
The structural difference with DNA is that RNA contains a -OH group both at the 2' and 3' position of the ribose ring, whereas DNA (which stands, in fact, for deoxy-RNA) lacks such a hydroxy group at the 2' position of the ribose. The same bases can be attached to the ribose group in RNA as occur in DNA, with the exception that in RNA thymine does not occur, and is replaced by uracil, which has an H-group instead of a methyl group at the C-5 position of the pyrimidine. Unlike the double-stranded DNA molecule, RNA is a single-stranded molecule.
The three main functionally distinct varieties of RNA molecules are: (1) messenger RNA (mRNA) which is involved in the transmission of DNA information, (2) ribosomal RNa (rRNA) which makes up the physical machinery of the synthetic process, and (3) transfer RNA (tRNA) which also constitutes another functional part of the machinery of protein synthesis.
acid dissociation constant → konstanta disocijacije kiseline
Acid dissociation constant (Ka) is the equilibrium constant for the dissociation of an acid HA through the reaction
The quantity pKa = -log Ka is often used to express the acid dissociation constant.
carboxylic acids → karboksilne kiseline
Carboxylic acids are organic compounds characterized by the presence of one or more RC(=O)OH groups (the carboxyl group). In the systematic chemical nomenclature carboxylic acids names end in the suffix -oic (e.g. ethanoic acids, CH3COOH). The carbon of the terminal group being counted as part of the chain. They are generally weak acids. Carboxylic acids include a large and important class of fatty acids and may be either saturated or unsaturated. There are also some natural aromatic carboxylic acids (benzoic, salicylic).
polyprotonic acids → poliprotonske kiseline
Polyprotonic acids are acids which dissolve in more than one degree.
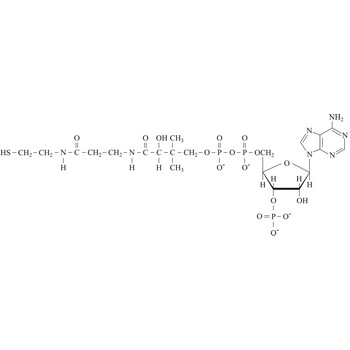
coenzyme a → koenzim a
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
glyceride → glicerid
Glycerides are esters of glycerol (propane-1,2,3-triol) with fatty acids, widely distributed in nature. They are by a long-established custom subdivided into triglycerides, 1,2- or 1,3-diglycerides, and 1- or 2- monoglycerides, according to the number and positions of acyl groups.
Citing this page:
Generalic, Eni. "Masna kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table