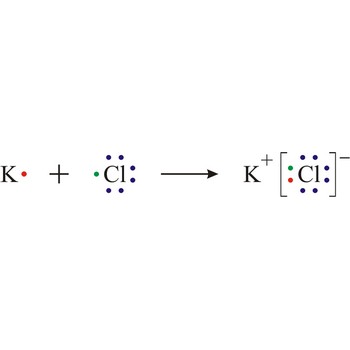Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
ligand → ligand
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
liquid aggregate state → tekuće agregatno stanje
Liquids have a constant volume, but they do not have a constant shape. They assume the shape of a vessel they are in at the moment. It happens because molecules of liquids are still close enough to each other, the attracting forces still being very strong.
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
lysine → lizin
Lysine is an electrically charged amino acids with basic side chains. Lysine is a base, as are arginine and histidine. The amino group is highly reactive and often participates in reactions at the active centers of enzymes. Lysine plays an important role in coordinating negatively charged ligands. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Lys, K
- IUPAC name: 2,6-diaminohexanoic acid
- Molecular formula: C6H14N2O2
- Molecular weight: 146.19 g/mol
macromolecule → makromolekule
Macromolecule is a molecule of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetitions of units derived, actually or conceptually, from molecules of low relative molecular mass. The types of macromolecules are natural and synthetic polymers, carbohydrates, lipids, proteins etc. Cellulose is a polysaccharide that is made up of hundreds, even thousands of glucose molecules strung together.
mass spectrometry → masena spectrometrija
Mass spectrometry is an analytical technique in which ions are separated according to the mass/charge (m/e) ratio and detected by a suitable detector.
In a mass spectrometer a sample is ionised and the positive ions produced are accelerated into a high-vacuum region containing electric and magnetic fields. These fields deflect and focus the ions onto a detector. A mass spectrum is thus obtained, consisting of a series of peaks of variable intensity to which m/e values can be assigned. Different molecules can be identified by their characteristic pattern of lines.
methionine → metionin
Methionine is neutral amino acids with polar side chains. It is one of the two sulfur-containing amino acids. Methionine is a fairly hydrophobic amino acid and typically found buried within the interior of a protein. It can form stacking interactions with the aromatic moieties of tryptophan, phenylalanine, and tyrosine. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Met, M
- IUPAC name: 2-amino-4-methylsulfanylbutanoic acid
- Molecular formula: C5H11NO2S
- Molecular weight: 149.21 g/mol
molar mass → molarna masa
Molar mass is the mass of one mole of a substance.
It is normally expressed in units of g mol-1, in which case its numerical value is identical with the relative molecular mass.
Citing this page:
Generalic, Eni. "Kvadratna piramidalna geometrija molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table