Rayleigh number → Rayleighova značajka
Rayleigh number (Ra) is a dimensionless quantity used in fluid mechanics, defined by
where l is length, g is acceleration of gravity, α is cubic expansion coefficient, T is temperature, ρ is density, η is viscosity, and a is thermal diffusivity.
refractometer → refraktometar
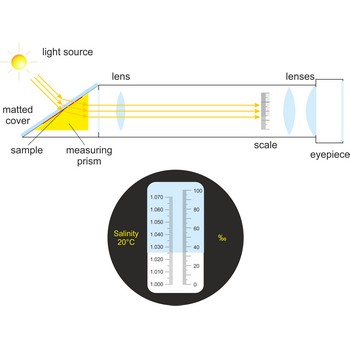
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
relative density → relativna gustoća
Relative density (d) is the ratio of the density of a substance to the density of some reference substance. For liquids or solids it is the ratio of the density (usually at 20 °C) to the density of water at 4 °C. Since one must specify the temperature of both the sample and the water to have a precisely defined quantity, the use of this term is now discouraged. This quantity was formerly called specific gravity.
reversible process → reverzibilan proces
Reversible process or reaction is those that can be reversed by an infinitesimally small change in conditions. For example, ice and water coexist at 101 325 Pa and 0 °C; a very slight temperature increase causes the ice to melt; a tiny temperature decrease causes the water to freeze. Melting or freezing under these conditions can be considered reversible.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
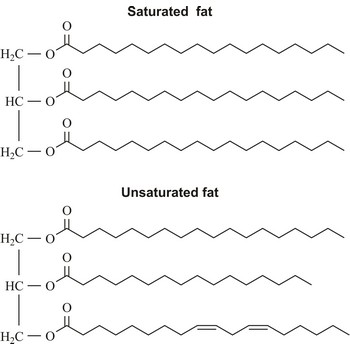
saturated fat → zasićena mast
Saturated fats are fats in foods that are solid at room temperature. They come chiefly from animal sources (beef, whole-milk dairy products, dark meat poultry) but also from tropical vegetable oils (coconut, palm).
saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
semiconductor → poluvodič
Semiconductor is a material in which the highest occupied energy band (valence band) is completely filled with electrons at T = 0 K, and the energy gap to the next highest band (conduction band) ranges from 0 to 4 or 5 eV. With increasing temperature electrons are excited into the conduction band, leading to an increase in the electrical conductivity.
silicon → silicij
Silicon was discovered by Jöns Jacob Berzelius (Sweden) in 1824. The origin of the name comes from the Latin word silicis meaning flint. Amorphous form of silicon is brown powder; crystalline form has grey metallic appearance. Solid form unreactive with oxygen, water and most acids. Dissolves in hot alkali. Silica dust is a moderately toxic acute irritant. Silicon makes up major portion of clay, granite, quartz and sand. Commercial production depends on a reaction between sand (SiO2) and carbon at a temperature of around 2200 °C. Used in glass as silicon dioxide (SiO2). Silicon carbide (SiC) is one of the hardest substances known and used in polishing. Also the crystalline form is used in semiconductors.
Citing this page:
Generalic, Eni. "Kritična temperatura." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table