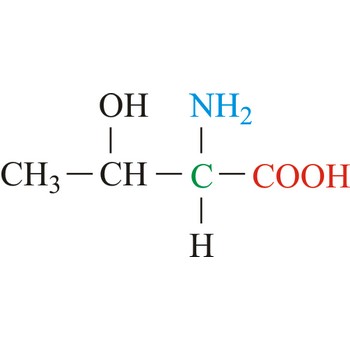
chiral centre → kiralno središte
Chiral centre in organic chemistry is most often an asymmetrically substituted carbon atom (C*).
chiral molecule → kiralne molekule
Chiral molecule is a molecule which cannot be superimposed on its mirror image. A common example is an organic molecule containing a carbon atom to which four different atoms or groups are attached. Such molecules exhibit optical activity, i.e., they rotate the plane of a polarised light beam.
threonine → treonin
Threonine is neutral amino acids with polar side chains. It differs from serine by having a methyl substituent in place of one of the hydrogens on the β carbon. Threonine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Thr, T
- IUPAC name: 2-amino-3-hydroxybutanoic acid
- Molecular formula: C4H9NO3
- Molecular weight: 119.12 g/mol
angular velocity → kutna brzina
A point-like object that undergoes circular motion changes its angular position from initial Θi to final Θf, relative to a fixed axis, specified in a coordinate system with an origin that coincides the centre of the circular path of object. The change in its angular position is called angular displacement ΔΘ = Θf - Θi. Also, a rigid body that rotates about a specified rotation axis, changing its angular position from initial Θi to final Θf, undergoes an angular displacement ΔΘ.
The average angular velocity, ωav, is the ratio of the angular displacement and the time interval Δt=tf-ti, in which that displacement occurs.
Θf and Θi are the initial and final angular position, respectively.
The instantaneous angular velocity ω is the limit of the average angular velocity, as Δt is made to approach zero.
ωav and ω are positive for the counterclockwise rotation (in direction of increasing Θ) and negative for the clockwise rotation (in direction of decreasing Θ).
SI unit for angular velocity is s-1.The measure for the angle Θ is radian. The relationship between radians and degrees is:
For example, the angular velocity of the minute hand of a clock is:
centrifugal force → centrifugalna sila
Centrifugal force is a force which moves a body away from the centre of motion.
micelle → micele
Micelle is an electrically charged colloidal particle, usually organic in nature, composed of aggregates of large molecules, e.g., in soaps and surfactants. For aqueous solutions, the hydrophilic end of the molecule is on the surface of the micelle, while the hydrophobic end (often a hydrocarbon chain) points toward the centre.
stereospecific reaction → stereospecifična reakcija
Stereospecific reactions are reactions that proceed predominantly to a single stereoisomeric product out. All metabolic conversions involving chiral molecules are stereospecific.
diastereoisomer → dijastereoizomer
Diastereoisomers (diastereomers) are stereoisomers of a compound having two or more chiral centers that are not a mirror image of another stereoisomer of the same compound. For example, in the structure below, 1 and 2 are enantiomers and so are 3 and 4; 1 and 3 are diastereoisomers, as are 2 and 4. Unlike enantiomers, diastereoisomers need not have closely similar physical and chemical properties
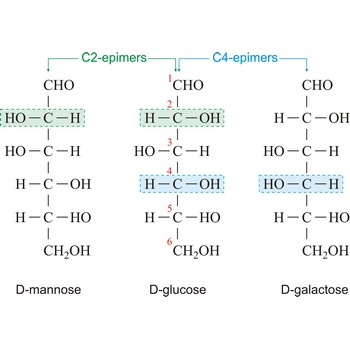
epimer → epimer
Epimers are diastereoisomers that have the opposite configuration at only one of two or more chiral centers present in the respective molecular entities. For example D-glucose and D-mannose, which differ only in the stereochemistry at C-2, are epimers, as are D-glucose and D-galactose (which differ at C-4).
hexagonal lattice → heksagonska rešetka
Hexagonal lattice has lattice points at the twelve corners of the hexagonal prism and at the centers of the two hexagonal faces of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=90° and γ=120°.
Citing this page:
Generalic, Eni. "Kiralno središte." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table