carbohydrate → ugljikohidrat
Carbohydrates (often called carbs for short) are polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. They are also known as saccharides, a term derived from the Latin word saccharum for sugar. Carbohydrates are the most abundant class of compounds in the biological world, making up more than 50 % of the dry weight of the Earth’s biomass. Every type of food we eat can have its energy traced back to a plant. Plants use carbon dioxide and water to make glucose, a simple sugar, in photosynthesis. Other carbohydrates such as cellulose and starch are made from the glucose. Light from the sun is absorbed by chlorophyll and this is converted to the energy necessary to biosynthesize carbohydrates
The term carbohydrate was applied originally to monosaccharides, in recognition of the fact that their empirical composition can be expressed as Cx(H2O)y. Later structural studies revealed that these compounds were not hydrates but the term carbohydrate persists.
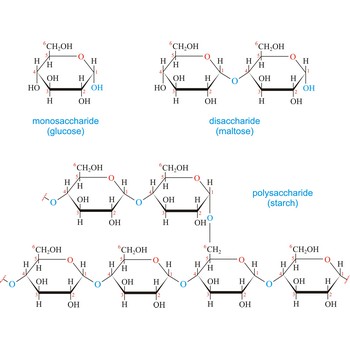
Carbohydrates are generally classed as either simple or complex. Simple sugars, or monosaccharides, are carbohydrates that can’t be converted into smaller subunits by hydrolysis. Complex carbohydrates are made of two (disaccharides) or more (oligosaccharides, polysaccharides) simple sugars linked together by acetal (glycosidic) bonds and can be split into the former by hydrolysis.
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
covalentity → kovalentnost
Covalentity is a maximum number of covalent bond that one atom can make, it is equal to the number of hydrogen atoms that are combined with other atoms. It is usually constant.
cycloalkanes → cikloalkani
Cycloalkanes are cyclic saturated hydrocarbons containing a ring of carbon atoms joined by single bonds. They have the general formula CnH2n, for example cyclohexane, C6H12. In general, they behave like the alkanes but are rather less reactive.
cement → cement
Cement is any various substances used for bonding or setting to a hard material. Portland cement is a mixture of calcium silicates and aluminates made by heating limestone (CaCO3) with clay (containing aluminosilicates) in a kiln. The product is ground to a fine powder. When mixed with water it settles in a few hours and then hardens over a longer period of time due to the formation of hydrated aluminates and silicates.
chelate → kelat
Chelate is a compound characterized by the presence of bonds from two or more bonding sites within the same ligand to a central metal atom. For example, copper complexes with EDTA to form a chelate. Chelate complexes are more stable than complexes with the corresponding monodentate ligands.
chiral molecule → kiralne molekule
Chiral molecule is a molecule which cannot be superimposed on its mirror image. A common example is an organic molecule containing a carbon atom to which four different atoms or groups are attached. Such molecules exhibit optical activity, i.e., they rotate the plane of a polarised light beam.
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
dehydrogenation → dehidrogenacija
Dehydrogenation is a chemical reaction in which hydrogen is removed from a compound. Dehydrogenation of organic compounds converts single carbon-carbon bonds into double bonds. It is usually affected by means of a metal catalyst or in biological systems by enzyme dehydrogenases.
diazo compounds → diazo-spojevi
Diazo compounds are compounds having the divalent diazo group, =N+=N-, attached to a carbon atom. The term includes azo compounds, diazonium compounds, and also such compounds as diazomethane, CH2=N2.
Citing this page:
Generalic, Eni. "Ionska veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table