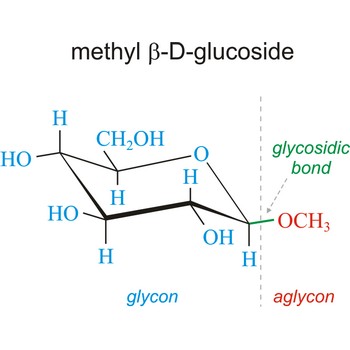
glycosidic bond → glikozidna veza
Glycosidic bond ia a bond between the glycosyl group, the structure obtained by removing the hydroxy group from the hemiacetal function of a monosaccharide, and the -OR group (which itself may be derived from a saccharide and chalcogen replacements thereof (RS–, RSe–). The terms N-glycosides and C-glycosides are misnomers and should not be used. The glycosidic bond can be α or β in orientation, depending on whether the anomeric hydroxyl group was α or β before the glycosidic bond was formed and on the specificity of the enzymatic reaction catalyzing their formation. Once the glycosidic bond is formed, the anomeric configuration of the ring is locked as either α or β. Specific glycosidic bonds therefore may be designated α(1→4), β(1→4), α(1→6), and so on. Cellulose is formed of glucose molecules linked by β(1→4)-glycosidic bonds, whereas starch is composed of α(1→4)-glycosidic bonds.
hydrogen bond → vodikova veza
Hydrogen is a bond formed by a hydrogen atom to an electronegative atom, and is denoted by dashed lines H-X---H-B. A hydrogen atom covalently bound to an oxygen (electronegative atom) has a significant positive charge and can form a weak bond to another electronegative atom.
ionic strength → ionska jakost otopine
Ionic strength (μ or I) is a measure of the total concentration of ions in a solution, defined by
where zi is the charge of ionic species i and ci is its concentration.
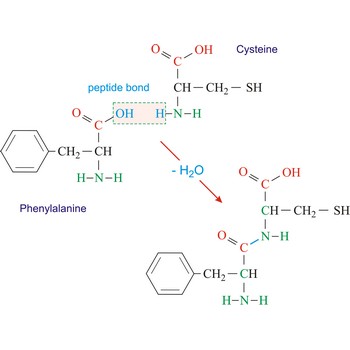
peptide bond → peptidna veza
Peptide bond emerges when two amino acid join in a way that the carbon atom from one connects with the nitrogen atom from the other (creating a C-N bond).
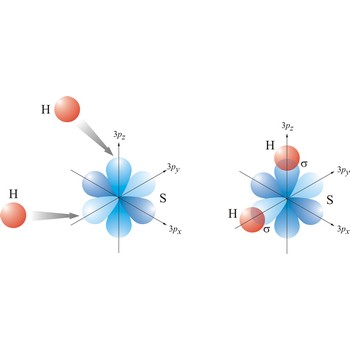
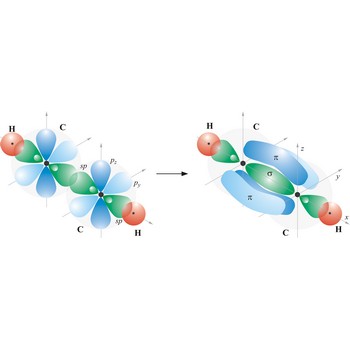
sigma bond → sigma veza
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
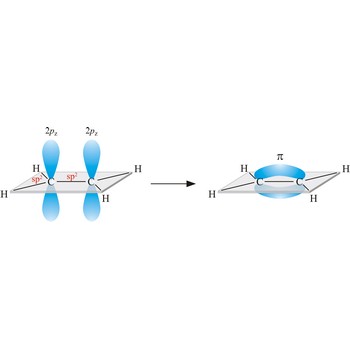
triple bond → trostruka veza
Triple bond. (≡) is a covalent bond that involves 3 bonding pairs. In the valence bond theory, one of the bonds in a triple bond is a sigma bond and the other two are pi bonds. For example, the central bond in acetylene is a triple bond: H-C≡C-H.
ion exchanger → ionski izmjenjivač
Ion-exchanger is a solid or liquid material containing ions that are exchangeable with other ions with a like charge that are present in a solution in which the material is insoluble. Ion-exchange resins consist of various copolymers having a cross-linked three-dimensional structure to which ionic groups have been attached.
ionic conductor → ionski vodič
Ionic conductor is a material that conducts electricity with ions as charge carriers.
Citing this page:
Generalic, Eni. "Ionska veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table