polyvalent molecule → polivalentna molekula
Polyvalent molecule is a molecule which having multiple binding sites. The antibodies of our immune system are one example.
fat → mast
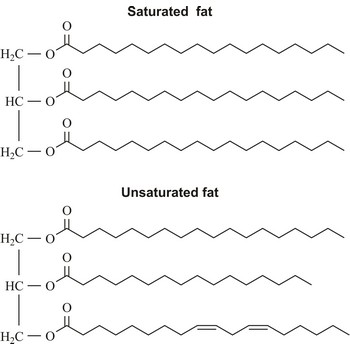
Fats are esters of glycerol and long chain carboxylic acids. Fats occur widely in plants and animals as a means of storing food energy, having twice the calorific value of carbohydrates. Fats derived from plants and fish generally have a greater proportion of unsaturated fatty acids than those from mammals. Fats may be either solid or liquid at room temperature, depending on their structure and composition. Unsaturated fats are liquid at room temperature.
Plant oils may be hardened by the addition of hydrogen atoms, converting double bonds to single bonds. This process is known as hydrogenation. Hydrogenated vegetable oils are often present in margarine and other processed foods.
Alkali hydrolysis of fat with sodium hydroxide it gives glycerol and soap (i.e. a mixture of the sodium salts of the fatty acids).
free radical → slobodni radikal
Free radical is a molecular fragment having one or more unpaired electrons, usually short-lived and highly reactive. They can be produced by photolysis or pyrolysis in which a bond is broken without forming ions. In formulas, a free radical is conventionally indicated by a dot (·CH3, ·SnH3, ·Cl). Free radicals are known to be formed by ionising radiation and thus play a part in deleterious degradation effects that occur in irradiated tissue. They also act as initiators or intermediates in oxidation, combustion, photolysis, and polymerisation.
fructose → fruktoza
Fructose (fruit sugar) is a ketohexose (a six-carbon ketonic sugar), which occurs in sweet fruits and honey. Glucose and fructose have the same molecular formula, C6H12O6, but have different structures. Pure, dry fructose is a very sweet, white, odorless, crystalline solid. Fructose is one of the sweetest of all sugars and is combined with glucose to make sucrose, or common table sugar. An older common name for fructose is levulose, after its levorotatory property of rotating plane polarized light to the left (in contrast to glucose which is dextrorotatory). The polysaccharide inulin is a polymer of fructose.
primary alcohol → primarni alkohol
Primary alcohols are alcohols where the hydroxyl group is attached to a primary carbon atom. Thus, it has the general structure, RCH2OH, where R is a hydrogen atom or an alkyl group.
primary amine → primarni amin
Primary amine is an amine on which there is only one alkyl group bonded, it is a weak base, and has a fish odour.
fuel cell → gorivi članak
Fuel cell is a device that converts chemical energy into electrical energy. It is different from a battery in that the energy conversion continues as long as fuel and oxidising agent are fed to the fuel cell; that is, in principle indefinitely. (A battery is manufactured with a limited amount of chemicals, and it is exhausted when all the chemicals have reacted.) It is a galvanic cell where spontaneous chemical reactions occur at the electrodes. The fuel is oxidised at the anode, and the oxidising agent (almost always oxygen or air) is reduced at the cathode. Presently, the most commonly used fuel is hydrogen. More conventional fuels (e.g., petrol or natural gas) must be converted (reformed) into hydrogen before they can be utilised in a fuel cell.
Some fuel cells employ an aqueous solution as electrolyte, that can be either acidic or basic (alkaline), or an ion-exchange membrane soaked in aqueous solution can act as the electrolyte. These fuel cells operate at relatively low temperatures (from room temperature to not much above the boiling point of water). Some fuel cells employ molten salts (especially carbonates) as electrolytes and have to operate at temperatures of several hundred degrees centigrade (Celsius). Others employ ionically conductive solids as electrolyte and must operate close to 1 000 °C.
Gauss’ law for electrostatics → Gaussov zakon za elektrostatiku
Gauss’ law describes the relation between charge and electric field in static situations, so it is equivalent to Coulomb’s law, which can be derived from Gauss’ law. Gauss’ law states that the net flux of electric field, Φ, through an imaginary closed surface, S, - a Gaussian surface - is equal to the net charge, q, inside that closed surface:
where electric flux Φ through Gaussian surface is given by:
ε0 is the permittivity constant and dS is a surface element.
regeneration → regeneracija
Regeneration is the process of restoring an ion exchange medium to a usable state after exhaustion. The cation exchanger is normally regenerated with hydrochloric acid and the anion exchanger with sodium hydroxide.
Citing this page:
Generalic, Eni. "Ionska veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table