allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
anhydrous → anhidrid
Anhydrous (without water) is an applied to minerals which do not contain water of crystallization or water of chemical combination. For example, strongly heated copper (II) sulphate pent hydrate (CuSO4•5H2O) produces anhydrous copper (II) sulphate (CuSO4). Less stable and more dangerous to use than hydrated.
argon → argon
Argon was discovered by Lord Raleigh and Sir William Ramsay (Scotland) in 1894. The origin of the name comes from the Greek word argos meaning inactive. It is colourless and odourless noble gas. Chemically inert. It is the third most abundant element in the earth’s atmosphere and makes up about 1 %. Argon is continuously released into the air by decay of radioactive potassium-40. Pure form is obtained from fractional distillation of liquid air. Used in lighting products. It is often used in filling incandescent light bulbs. Some is mixed with krypton in fluorescent lamps. Crystals in the semiconductor industry are grown in argon atmospheres.
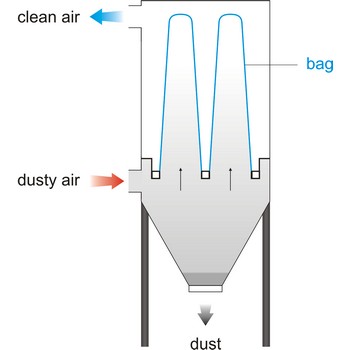
bag filter → vrećasti filtar
Bag filter is a unit within a mechanical system that bellows in a bag form when air flows through, cleaning the air by collecting particles of foreign matter. This system of filtering is rated the most efficient- in a range of 92 % to 95 %. Vacuum cleaner have a cloth filter bag.
Bag filter system is an economical method of liquid filtration consisting of the pressure vessel, restrainer basket and micron rated filter bag. Liquid flow is from the inside to the outside of the bag - dirt is trapped inside the bag.
chemical balance → kemijska ravnoteža
Chemical balance is a degree of reversible reaction in a closed system, when the forward and backward reaction happen at same rates and their effects annul each other, while the concentration of reactants and products stays the same.
combustion → gorenje
Combustion is a phenomenon when a substance is combined with oxygen in the presence of a flame accompanied by the production of heat and light. Combustion requires a supply of both fuel and oxygen (air) and can take place in the open atmosphere such as an open fire, or in a closed system, such as a car engine.
analytical balance → analitička vaga
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
barium → barij
Barium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word barys meaning heavy. It is soft, slightly malleable, silvery-white metal. Attacked by air and water. Soluble compounds toxic by ingestion. Barium is found in barytine (BaSO4) and witherite (BaCO3), never found in pure form due to its reactivity. Must be stored under kerosene to remain pure. Barite, or barium sulfate (BaSO4), when ground is used as a filter for rubber, plastics and resins. It is insoluble in water and so is used in X-rays of the digestive system. Barium nitrate, Ba(NO3)2, burns brilliant green and is used in fireworks.
conjugated double bond → konjugirana dvostruka veza
Conjugated double bond in organic compounds is a system of double bonds between atoms which are separated by one single bond (1,3-butene, H2C=CH-CH=CH2).
Citing this page:
Generalic, Eni. "Hexagonal_crystal_system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table