psychoactive drug → psihoaktivna droga
Psychoactive drugs are natural (mescaline) or synthetic substances (LSD) which take effect on central nervous system causing euphoria, and by lengthened use they also cause addiction, gradually destroying the nervous system.
purification → pročišćavanje
Purification is the physical or chemical process of removing contaminants from a compound. The physical processes may include sublimation, distillation, filtration, crystallisation, or extraction. The chemical processes may involve formation of a derivative, purification of the derivative and recovery of the original material in a pure form of the derivative.
heat capacity → toplinski kapacitet
Heat capacity is defined in general as dQ/dT, where dQ is the amount of heat that must be added to a system to increase its temperature by a small amount dT. The heat capacity at a constant pressure is Cp = (∂H/∂T)p; that at a constant volume is CV = (∂E/∂T)V, where H is enthalpy, E is internal energy, p is pressure, V is volume, and T is temperature. An upper case C normally indicates the molar heat capacity, while a lower case c is used for the specific (per unit mass) heat capacity.
Hesse’s law → Hessov zakon
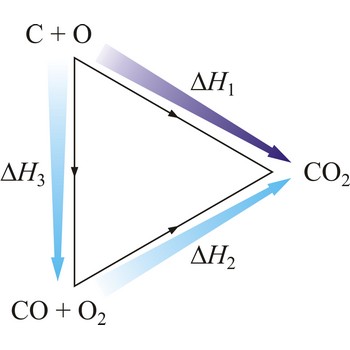
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
quasicrystal → kvazikristal
Quasicrystal is a solid having conventional crystalline properties but whose lattice does not display translational periodicity.
recrystallisation → rekristalizacija
Recrystallisation is a formation of a new, strain free grain structure from the existing one in cold worked metal, usually accomplished by heating. The change from one crystal structure to another, as occurs on heating or cooling through a critical temperature.
resonant frequency → rezonantna frekvencija
All vibrating systems have one or more resonant frequencies, which depend on system characteristics. If an external force is applied on the system at that frequency, the vibrations will be much greater than at slight different frequencies.
Citing this page:
Generalic, Eni. "Hexagonal_crystal_system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table