pigment → pigment
Pigments are the substances that give paint colour. Pigments are derived from natural or synthetic materials that have been ground into fine powders. A pigment is different from a dye in that a pigment is insoluble in the media in which it is used.
Pigment is an organic substance found in plant and animal cells that creates colouring.
positive pole → pozitivni pol
Positive pole is that half-cell in the electrochemical cell which has the most positive electrode potential.
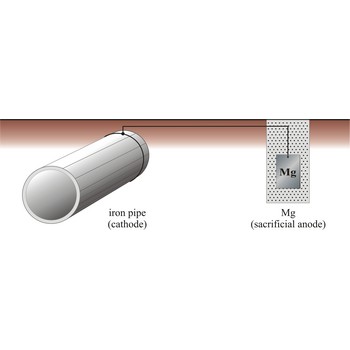
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
specific heat → specifični toplinski kapacitet
Specific heat is the quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius.
Charles’ law → Charlesov zakon
The volume of a fixed mass of gas at a constant pressure expand by the constant fraction of its volume at 0 °C. For each Celsius or kelvin degree its temperature is raised. For any ideal gas fraction it is approximately 1/273. This can be expressed by the equation
were V° is the volume at 0°C and V is its volume at t°C.
This is equivalent to the statement that the volume of a fixed mass of gas at a constant pressure is proportional to its thermodynamic temperature
This law also know as Gay-Lussac’s law.
An equation similar to the one given above applies to pressures for ideal gases:
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
coulometer → kulometar
Coulometer is a type of electrolysis cell which is used for measuring the quantity of one element released during electrolysis.
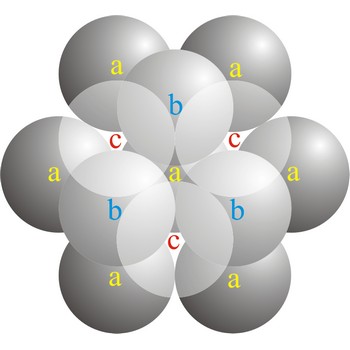
cubic close-packed structure → kubična gusta slagalina
In a cubic close-packed (ccp) arrangement of atoms, the unit cell consists of four layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The atoms in the second layer (b) fit into depressions in the first layer. The atoms in the third layer (c) occupy a different set of depressions than those in the first. The cubic close packed structure can be made by piling layers in the a-b-c-a-b-c-a-b-c... sequence.
degree → stupanj
1. Degree is a unit of temperature on a specified scale. The temperatures of boiling and freezing water are: in the Fahrenheit system 212 and 32 degrees; in the Celsius system 100 and 0 (zero) degrees.
2. Degree is a unit of angular measure. A circle is divided into 360 degrees, represented by the symbol °. Degrees are each divided into 60 minutes. Each minute has 60 seconds. Symbols for degree, minute, and second for plane angle is placed after the numerical value and a no space between the numerical value and the unit symbol (α = 2°3'4").
3. In algebra, the degree of a polynomial is the highest power of the variable in the polynomial. For example, 4x3 + 3x2 + x + 7 have degree 3.
deoxyribonucleic acid → dezoksiribonukleinska kiselina
Deoxyribonucleic acid (DNA) is a nucleic acid with 2-deoxy-D-ribose as the sugar in its nucleotides. DNA contains encoded genetic information, specifically templates for the synthesis of all of an organism’s proteins and enzymes.
DNA was first identified in the 1869 by Swiss chemist Friedrich Miescher (1844-1895). In 1953, American biologist James Dewey Watson (1928-) and English physicist Francis Harry Compton Crick (1916–2004) had discovered that DNA occurs in the cell as a double helix, with two long strands of the molecule wound around each other, and further that the chemical structure of the molecule dictates that adenine (A) always aligns or pairs with thymine (T), and cytosine (C) always pairs with guanine (G). It is this base pairing that allows DNA in a cell to copy itself, and transfer its information to a new cell. The diameter of the helix is 2.0 nm and there is a residue on each chain every 0.34 nm in the z direction. The angle between each residue on the same strand is 36°, so that the structure repeats after 10 residues (3.4 nm) on each strand.
Citing this page:
Generalic, Eni. "Galvanic cell." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table